Biomarker characterization in endometrial cancer in Italy: first survey data analysis
- PMID: 35775705
- PMCID: PMC9248238
- DOI: 10.32074/1591-951X-775
Biomarker characterization in endometrial cancer in Italy: first survey data analysis
Abstract
Objective: Endometrial carcinoma (EC) is the most common gynecological malignant disease in high income countries. The 2020 edition of the World Health Organization (WHO) Classification of Tumors of the Female Genital Tract underlines the important clinical implications of the new integrated histo-molecular classification system, in order to correctly define the specific prognostic risk group. This survey analysis will focus on the most commonly adopted immunohistochemical and molecular biomarkers used in daily clinical characterization of a diagnosed endometrial carcinoma in Italian labs.
Methods: An evaluation questionnaire was distributed to 41 Italian pathology laboratories. Normal habits in EC evaluation, especially regarding mismatch repair status (MMR) and microsatellite instability (MSI), were collected. A summary and a descriptive statistical analysis were used to show the current practice of each laboratory.
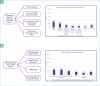
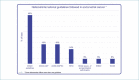
Results: The analysis of MMR status by immunohistochemistry (IHC) is carried out on the majority of all EC samples. The most frequent strategy for the analysis of MMR status in EC is IHC of four proteins (PMS2, MSH6, MSH2, MLH1). MSI analysis by molecular method in endometrial cancer is rarer and more restricted to some circumstances. Hypermethylation of the MLH1 promoter by methylation-specific PCR and pyrosequencing was analyzed in case of negative expression of MLH1/PMS2. Also, the analysis of p53 in EC is performed in the majority of cases. POLE mutational profiling is adopted only in a limited number of laboratories. Fifty-five percent of Italian laboratories refer to national/international guidelines when analyzing biomarkers in EC (among those, 45% use the ESGO Guidelines, 18% ASCO-CAP, 18% AIOM, 14% WHO, 5% British Association of Gynaecological Pathologist, 5% ESMO, 5% NCCN).
Conclusions: Adoption of guidelines and standardization of pre-analytical and analytical procedures are effective tools for adequate EC prognostic risk stratification and high quality standard of care.
Keywords: Italian labs; endometrial cancer (EC); guidelines; immunohistochemistry (IHC); molecular analysis.
Copyright © 2022 Società Italiana di Anatomia Patologica e Citopatologia Diagnostica, Divisione Italiana della International Academy of Pathology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Cancer Genome Atlas Research Network. Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013;497(7447):67-73. https://doi.org/10.1038/nature12113. 10.1038/nature12113 Erratum in: Nature 2013;500(7461):242. - DOI - PMC - PubMed
-
- Talhouk A, McConechy MK, Leung S, et al. Confirmation of ProMisE: a simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017;123:802-813. https://doi.org/10.1002/cncr.30496. 10.1002/cncr.30496 Epub 2017 Jan 6. - DOI - PubMed
-
- Kommoss S, McConechy MK, Kommoss F, et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann Oncol 2018;29:1180-1188. https://doi.org/10.1093/annonc/mdy058 10.1093/annonc/mdy058 - DOI - PubMed
-
- Stelloo E, Nout RA, Osse EM, et al. Improved Risk Assessment by Integrating Molecular and Clinicopathological Factors in Early-stage Endometrial Cancer – Combined Analysis of the PORTEC Cohorts. Clin Cancer Res 2016;22:4215-4224. https://doi.org/10.1158/1078-0432.CCR-15-2878 10.1158/1078-0432.CCR-15-2878 - DOI - PubMed
-
- Concin N, Matias-Guiu X, Vergote I, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer 2021;31:12-39. https://doi.org/10.1136/ijgc-2020-002230. 10.1136/ijgc-2020-002230 Epub 2020 Dec 18. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

