A novel platform for attenuating immune hyperactivity using EXO-CD24 in COVID-19 and beyond
- PMID: 35776000
- PMCID: PMC9349550
- DOI: 10.15252/emmm.202215997
A novel platform for attenuating immune hyperactivity using EXO-CD24 in COVID-19 and beyond
Abstract
A small but significant proportion of COVID-19 patients develop life-threatening cytokine storm. We have developed a new anti-inflammatory drug, EXO-CD24, a combination of an immune checkpoint (CD24) and a delivery platform (exosomes). CD24 inhibits the NF-kB pathway and the production of cytokines/chemokines. EXO-CD24 discriminates damage-from pathogen-associated molecular patterns (DAMPs and PAMPs) therefore does not interfere with viral clearance. EXO-CD24 was produced and purified from CD24-expressing 293-TREx™ cells. Exosomes displaying murine CD24 (mCD24) were also created. EXO-CD24/mCD24 were characterized and examined, for safety and efficacy, in vitro and in vivo. In a phase Ib/IIa study, 35 patients with moderate-high severity COVID-19 were recruited and given escalating doses, 108 -1010 , of EXO-CD24 by inhalation, QD, for 5 days. No adverse events related to the drug were observed up to 443-575 days. EXO-CD24 effectively reduced inflammatory markers and cytokine/chemokine, although randomized studies are required. EXO-CD24 may be a treatment strategy to suppress the hyper-inflammatory response in the lungs of COVID-19 patients and further serve as a therapeutic platform for other pulmonary and systemic diseases characterized by cytokine storm.
Keywords: CD24; COVID-19; EXO-CD24; cytokine storm; exosomes.
© 2022 The Authors. Published under the terms of the CC BY 4.0 license.
Figures

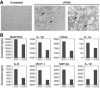
- A
Representative clones screened for CD24 expression under regulation of tetracycline. The cells were exposed to 1 μg/ml tetracycline for 48 h. Twenty microgram from each sample were subjected to Western blot analysis for CD24 and analyzed with anti‐CD24 SWA11 mAb. The membrane was reprobed with anti‐tubulin antibody to assess the uniformity of sample loading.
- B
The effect of tetracycline concentration on CD24 expression levels was examined by flow cytometry.
- C
Exosome‐based ELISA was used to measure expression of CD24 in the purified exosomes.
- D
Western blot analysis of EXO‐CD24 exosomes. Tetracycline (Tet) was used to induce CD24 expression in the cell line from which exosomes are derived. HSP70 was used as an exosomal marker.
- E
Flow cytometry analysis of EXO‐CD24. Left panel: Cells were gated based on cell size (forward scatter [FSC] versus side scatter [SSC]); Beads of known sizes of 100, 200 and 500 nm were used for size reference; Middle‐left panel: EXO‐CD24 labeled with anti‐CD24 FITC antibody and gated to the exosome population; Middle‐right panel: EXO‐CD24 labeled with anti‐CD81 APC antibody and gated by to the exosome population; Right panel: EXO‐CD24 double labeled with anti‐CD24 FITC and anti‐CD81 APC antibodies and gated by to the exosome population.
- F
Cryo‐TEM images of isolated CD24‐exosomes. On a number of different devices, many experiments were conducted and representative images are shown. Arrows point to double‐membraned vesicles (exosomes). Scale bars are 100 nm (left panel) and 200 nm (right panel).
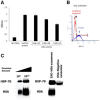
- A
PMA stimulation of monocyte cell line U937 show change in cell morphology and adherence. Untreated (left) and stimulated cells with PMA for 72 h (right). The arrows point to U937 macrophage‐like cells (scale bar ×10).
- B
U937 cells were stimulated with PMA and incubated with (+EXO) or without (−EXO) EXO‐CD24 treatment. Incubation medium was then collected and analyzed for cytokines/chemokines levels using a “Multi‐plex” array. Data shown are the average of duplicates from a single experiment.

- A
Daily body weights (gram). Body weight was measured daily during the first week and three times a week during the second week. Data are average ± SEM.
- B
Organ weight following a full 5‐day treatment course; B: brain, H: heart, Lu: lungs, T: thymus, S: spleen, K: kidneys, Li: liver. Data are average ± SEM.
- C
Urine analysis. The urine was collected and analyzed and no abnormalities were found.
- D
Hematology. Mice were anesthetized and blood was taken for full hematology tests. Data are average ± SEM.
- E
Blood Chemistry. Mice were anesthetized and blood was taken for clinical chemistry tests. Data are average ± SEM.
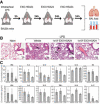
- A
Exosome‐based ELISA was used to measure expression of mCD24 in the purified exosomes. The expression level was compared to mCD24‐negative exosomes and to mouse recombinant protein. The data are represented as average of three technical replicates ± SEM.
- B
Flow cytometry analysis of EXO‐mCD24. EXO‐mCD24 labeled with APC‐conjugated anti‐mCD24 antibody and gated to the exosome population.
- C
Western blot analysis of EXO‐mCD24 exosomes. HSP70 was used as an exosomal marker.
- A
Study design. Animals were challenged by intratracheal introduction of LPS, followed by EXO‐HSA treatment, at 1 × 108 or 1 × 109 EXO‐HSA/mouse, once daily for 3 days and then, mice were sacrificed. Serum and BAL fluid (Bronchial alveolar lavage fluid) were collected.
- B
Histopathology. Representative histological images of lung tissue. Tissues from saline and 1 × 108 EXO‐HSA/mouse (low dose) treatment show extensive neutrophil infiltrate in the alveolar spaces (arrows) and around the bronchi and blood vessels (small arrows). In comparison, the inflammatory infiltrate in the 1 × 109 EXO‐HSA/mouse (high dose) treatment is considerably attenuated. All images: hematoxylin and eosin (H&E) staining (naïve: no LPS challenge).
- C
The levels of systemic and local cytokines and chemokines. Serum (upper panel) and BAL fluid (lower panel) cytokines/chemokines levels. (KC/CXCL1, a neutrophil chemokine). Data are average ± SEM, n = 10 mice/group.
- A
Cecum ligation and puncture (CLP) was performed, followed by inhalation treatment with vehicle, or 1 × 109, 1 × 1010, 1 × 1011 EXO HSA/mouse, at 1, 8 and 24 h post‐surgery.
- B, C
EXO‐CD24 was isolated from BALF (B) and serum (C) of treated and control mice. Evaluation of CD24/CD81‐positive exosomes, at each time point, was carried out by flow cytometry. The data are presented as average (n = 4 mice at each time point) ± SEM.
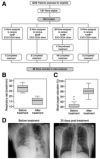
- A
One trial participant, in the 1 × 109 EXO‐CD24/dose group discontinued the treatment after 3 days due to sustained low blood oxygen saturation.
- B, C
(B) respiratory rate and (C) Blood oxygen (SpO2) and before treatment (Day 1, measured before administration of first dose) and after treatment (Day 7). Descriptive statistics (minimum, maximum, median, first quartile, third quartile) were calculated for Blood oxygen (SpO2) and respiratory rate. These are presented graphically via box plots before and after treatment.
- D
Representative X‐ray image.

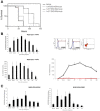
- A
Systemic CRP levels. Values are normalized against baseline value (Day 0). Descriptive statistics (minimum, maximum, median, first quartile, and third quartile) were calculated for CRP levels. These are presented graphically via box plots before and after treatment (n = 35 patients).
- B
Systemic cytokine levels. Cytokine levels were measured during (Day 3/4) or after (Day 7/8) treatment with EXO‐CD24 using Quantibody Multiplex ELISA Array. Values are normalized against baseline value (Day 0). Samples from 11 patients were analyzed. Results from eight patients are shown (results for three patients are not shown because one or more baseline results were below detection level). Box plot of Systemic cytokine levels by days after treatment (minimum, maximum, median, first quartile, third quartile).
- C
During the course of EXO‐CD24 treatment and follow‐up, cytokine levels were measured for the rest of the patients (n = 24 patients). Values represent the fold change from day 0, mean ± SEM.
- D
Monitoring systemic IL‐6 cytokine level following EXO‐CD24 treatment. Samples from 17 patients, representing the four experimental groups, were analyzed using the BD™ Cytometric Bead Array (CBA). Values represent mean ± SD.
References
-
- Al‐Ani B, ShamsEldeen AM, Kamar SS, Haidara MA, Al‐Hashem F, Alshahrani MY, Al‐Hakami AM, Kader DHA, Maarouf A (2022) Lipopolysaccharide induces acute lung injury and alveolar haemorrhage in association with the cytokine storm, coagulopathy and AT1R/JAK/STAT augmentation in a rat model that mimics moderate and severe Covid‐19 pathology. Clin Exp Pharmacol Physiol 49: 483–491 - PubMed
-
- Bradley CA (2019) CD24 – a novel 'don't eat me' signal. Nat Rev Cancer 19: 541 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

