Cancer-targeted photoimmunotherapy induces antitumor immunity and can be augmented by anti-PD-1 therapy for durable anticancer responses in an immunologically active murine tumor model
- PMID: 35776159
- PMCID: PMC9813181
- DOI: 10.1007/s00262-022-03239-9
Cancer-targeted photoimmunotherapy induces antitumor immunity and can be augmented by anti-PD-1 therapy for durable anticancer responses in an immunologically active murine tumor model
Abstract
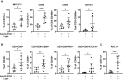
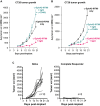
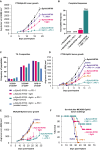
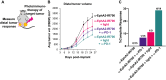
The complex immunosuppressive nature of solid tumor microenvironments poses a significant challenge to generating efficacious and durable anticancer responses. Photoimmunotherapy is a cancer treatment strategy by which an antibody is conjugated with a non-toxic light-activatable dye. Following administration of the conjugate and binding to the target tumor, subsequent local laser illumination activates the dye, resulting in highly specific target cell membrane disruption. Here we demonstrate that photoimmunotherapy treatment elicited tumor necrosis, thus inducing immunogenic cell death characterized by the release of damage-associated molecular patterns (DAMPs). Photoimmunotherapy-killed tumor cells activated dendritic cells (DC), leading to the production of proinflammatory cytokines, T cell stimulation, priming antigen-specific T cells, and durable memory T cell responses, which led complete responder mice to effectively reject new tumors upon rechallenge. PD-1 blockade in combination with photoimmunotherapy enhanced overall anticancer efficacy, including against anti-PD-1-resistant tumors. The combination treatment also elicited abscopal anticancer activity, as observed by reduction of distal, non-illuminated tumors, further demonstrating the ability of photoimmunotherapy to harness local and peripheral T cell responses. With this work we therefore delineate the immune mechanisms of action for photoimmunotherapy and demonstrate the potential for cancer-targeted photoimmunotherapy to be combined with other immunotherapy approaches for augmented, durable anticancer efficacy. Moreover, we demonstrate responses utilizing various immunocompetent mouse models, as well as in vitro data from human cells, suggesting broad translational potential.
Keywords: Cancer; Immuno-oncology; Immunology; Photoimmunotherapy.
© 2022. The Author(s).
Conflict of interest statement
This research was funded by Rakuten Medical, Inc (RMI). All authors are currently, or were employed by RMI, at the time of research. SMO, DMB, JJF, RH, MGG, MW, CDMF, and DY hold stock options at RMI. MGG, JJF, RH, CDMF, and DY hold IP interests at RMI. MW participates in biopharma consulting activities outside of RMI. MGG serves a leadership role at Rakuten Medical and holds IP interests at UC Irvine and RMI.
Figures



References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

