Selective neural coding of object, feature, and geometry spatial cues in humans
- PMID: 35776524
- PMCID: PMC9812241
- DOI: 10.1002/hbm.26002
Selective neural coding of object, feature, and geometry spatial cues in humans
Abstract
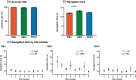
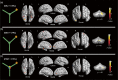
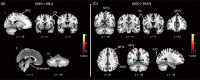
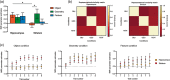
Orienting in space requires the processing of visual spatial cues. The dominant hypothesis about the brain structures mediating the coding of spatial cues stipulates the existence of a hippocampal-dependent system for the representation of geometry and a striatal-dependent system for the representation of landmarks. However, this dual-system hypothesis is based on paradigms that presented spatial cues conveying either conflicting or ambiguous spatial information and that used the term landmark to refer to both discrete three-dimensional objects and wall features. Here, we test the hypothesis of complex activation patterns in the hippocampus and the striatum during visual coding. We also postulate that object-based and feature-based navigation are not equivalent instances of landmark-based navigation. We examined how the neural networks associated with geometry-, object-, and feature-based spatial navigation compared with a control condition in a two-choice behavioral paradigm using fMRI. We showed that the hippocampus was involved in all three types of cue-based navigation, whereas the striatum was more strongly recruited in the presence of geometric cues than object or feature cues. We also found that unique, specific neural signatures were associated with each spatial cue. Object-based navigation elicited a widespread pattern of activity in temporal and occipital regions relative to feature-based navigation. These findings extend the current view of a dual, juxtaposed hippocampal-striatal system for visual spatial coding in humans. They also provide novel insights into the neural networks mediating object versus feature spatial coding, suggesting a need to distinguish these two types of landmarks in the context of human navigation.
Keywords: functional MRI; geometry; hippocampus; landmark; navigation; spatial cues; striatum.
© 2022 The Authors. Human Brain Mapping published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Bar, M. , Kassam, K. S. , Ghuman, A. S. , Boshyan, J. , Schmidt, A. M. , Dale, A. M. , Hämäläinen, M. S. , Marinkovic, K. , Schacter, D. L. , Rosen, B. R. , & Halgren, E. (2006). Top‐down facilitation of visual recognition. Proceedings of the National Academy of Sciences of the United States of America, 103, 449–454. 10.1073/pnas.0507062103 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

