Ablation of Refractory Ventricular Tachycardia Using Intramyocardial Needle Delivered Heated Saline-Enhanced Radiofrequency Energy: A First-in-Man Feasibility Trial
- PMID: 35776711
- PMCID: PMC9388560
- DOI: 10.1161/CIRCEP.121.010347
Ablation of Refractory Ventricular Tachycardia Using Intramyocardial Needle Delivered Heated Saline-Enhanced Radiofrequency Energy: A First-in-Man Feasibility Trial
Abstract
Background: Ablation of ventricular tachycardia (VT) is limited by the inability to create penetrating lesions to reach intramyocardial origins. Intramural needle ablation using in-catheter, heated saline-enhanced radio frequency (SERF) energy uses convective heating to increase heat transfer and produce deeper, controllable lesions at intramural targets. This first-in-human trial was designed to evaluate the safety and efficacy of SERF needle ablation in patients with refractory VT.
Methods: Thirty-two subjects from 6 centers underwent needle electrode ablation. Each had recurrent drug-refractory monomorphic VT after implantable cardioverter defibrillator implantation and prior standard ablation. During the SERF study procedure, one or more VTs were induced and mapped. The SERF needle catheter was used to create intramural lesions at targeted VT site(s). Acute procedural success was defined as noninducibility of the clinical VT after the procedure. Patients underwent follow-up at 30 days, and 3 and 6 months, with implantable cardioverter defibrillator interrogation at follow-up to determine VT recurrence.
Results: These refractory VT patients (91% male, 66±10 years, ejection fraction 35±11%; 56% ischemic, and 44% nonischemic) had a median of 45 device therapies (shock/antitachycardia pacing) for VT in the 3 to 6 months pre-SERF ablation. The study catheter was used to deliver an average of 10±5 lesions per case, with an average of 430±295 seconds of radiofrequency time, 122±65 minute of catheter use time, and a procedural duration of 4.3±1.3 hours. Acute procedural success was 97% for eliminating the clinical VT. At average follow-up of 5 months (n=32), device therapies were reduced by 89%. Complications included 2 periprocedural deaths: an embolic mesenteric infarct and cardiogenic shock, 2 mild strokes, and a pericardial effusion treated with pericardiocentesis (n=1).
Conclusions: Intramural heated saline needle ablation showed complete acute and satisfactory mid-term control of difficult VTs failing 1 to 5 prior ablations and drug therapy. Further study is warranted to define safety and longer-term efficacy.
Registration: URL: https://www.
Clinicaltrials: gov; Unique Identifier: NCT03628534 and NCT02994446.
Keywords: catheter; heating; pericardial effusion; shock, cardiogenic; tachycardia, ventricular.
Conflict of interest statement
Figures


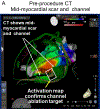





Comment in
-
A Force Awakens: Return of Needle Catheter Radiofrequency Ablation for Targeting Intramural Ventricular Arrhythmias.Circ Arrhythm Electrophysiol. 2022 Sep;15(9):e011309. doi: 10.1161/CIRCEP.122.011309. Epub 2022 Aug 25. Circ Arrhythm Electrophysiol. 2022. PMID: 36006297 No abstract available.
Similar articles
-
Evaluation of Saline-Enhanced Radiofrequency Needle-Tip Ablation for Ventricular Tachycardia (SERF VT CANADA Trial).Can J Cardiol. 2022 Aug;38(8):1277-1285. doi: 10.1016/j.cjca.2022.06.008. Epub 2022 Jun 15. Can J Cardiol. 2022. PMID: 35714882 Clinical Trial.
-
Initial human feasibility of infusion needle catheter ablation for refractory ventricular tachycardia.Circulation. 2013 Nov 19;128(21):2289-95. doi: 10.1161/CIRCULATIONAHA.113.003423. Epub 2013 Sep 13. Circulation. 2013. PMID: 24036605 Clinical Trial.
-
Long-Term Success of Irrigated Radiofrequency Catheter Ablation of Sustained Ventricular Tachycardia: Post-Approval THERMOCOOL VT Trial.J Am Coll Cardiol. 2016 Feb 16;67(6):674-683. doi: 10.1016/j.jacc.2015.11.041. J Am Coll Cardiol. 2016. PMID: 26868693 Clinical Trial.
-
Catheter Ablation of Ventricular Tachycardia in Patients With a Ventricular Assist Device: A Systematic Review of Procedural Characteristics and Outcomes.JACC Clin Electrophysiol. 2019 Jan;5(1):39-51. doi: 10.1016/j.jacep.2018.08.009. Epub 2018 Sep 26. JACC Clin Electrophysiol. 2019. PMID: 30678785
-
Clinical outcomes of radiofrequency catheter ablation of ventricular tachycardia in patients with hypertrophic cardiomyopathy.J Cardiovasc Electrophysiol. 2023 Jan;34(1):219-224. doi: 10.1111/jce.15739. Epub 2022 Nov 15. J Cardiovasc Electrophysiol. 2023. PMID: 36335616 Free PMC article.
Cited by
-
A Comprehensive Review on Stereotactic Arrhythmia Radioablation (STAR): Pioneering a New Era in Arrhythmia Management.Cureus. 2024 Oct 1;16(10):e70601. doi: 10.7759/cureus.70601. eCollection 2024 Oct. Cureus. 2024. PMID: 39483583 Free PMC article. Review.
-
What to do when everything fails…Is alcohol the answer?HeartRhythm Case Rep. 2022 Nov 11;9(1):6-7. doi: 10.1016/j.hrcr.2022.11.002. eCollection 2023 Jan. HeartRhythm Case Rep. 2022. PMID: 36685683 Free PMC article. No abstract available.
-
Intramural Ventricular Arrhythmias: How to Crack a Hard Nut.Curr Cardiol Rep. 2024 Dec;26(12):1405-1411. doi: 10.1007/s11886-024-02143-1. Epub 2024 Nov 27. Curr Cardiol Rep. 2024. PMID: 39602060 Free PMC article. Review.
-
Solving the Reach Problem: A Review of Present and Future Approaches for Addressing Ventricular Arrhythmias Arising from Deep Substrate.Arrhythm Electrophysiol Rev. 2023 Feb 16;12:e04. doi: 10.15420/aer.2022.28. eCollection 2023. Arrhythm Electrophysiol Rev. 2023. PMID: 37600155 Free PMC article. Review.
-
Ventricular Intramyocardial Navigation for Tachycardia Ablation Guided by Electrograms (VINTAGE): Initial Human Experience.JACC Clin Electrophysiol. 2025 Jul 7:S2405-500X(25)00462-1. doi: 10.1016/j.jacep.2025.06.003. Online ahead of print. JACC Clin Electrophysiol. 2025. PMID: 40704960
References
-
- Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC et al.: 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Circulation 2018;138:e272–e391. - PubMed
-
- Aliot E, Stevenson W, Almendral-Garrote J, Bogun F, Calkins H, Delacretaz E, Bella P, Hindricks G, Jais P, Josephson M et al.: EHRA/HRS Expert concensus on catheter ablation of ventricular arrhythmias: Developed in a partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA). Europace 2009,11:771–817. - PubMed
-
- Bunch T, Weiss J, Crandall B, Day J, May H, Bair T, Osborn J, C M, Fischer A, Brunner J et al.: Patients treated with catheter ablation for ventricular tachycardia after an ICD shock have lower long-term rates of death and heart failure hospitalization than do patients treated with medical management only. Heart Rhythm 2014;11:533–540. - PubMed
-
- Garcia F, Bazan V, Zado E, Ren J, Marchlinski F: Epicardial substrate and outcome with epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation 2009;120:366–375. - PubMed
-
- Natale A, Raviele A, Al-Ahmad A, Alfieri O, Aliot E, Almendral J, Breithardt G, Brugada J, Calkins H, Callans D et al.: Venice Chart International Consensus Document on ventricular tachycardia/ventricular filbrillation ablation. J Cardiovasc Electrophysiol 2010;21:339–379. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

