Integration of bioassay and non-target metabolite analysis of tomato reveals that β-carotene and lycopene activate the adiponectin signaling pathway, including AMPK phosphorylation
- PMID: 35776737
- PMCID: PMC9249195
- DOI: 10.1371/journal.pone.0267248
Integration of bioassay and non-target metabolite analysis of tomato reveals that β-carotene and lycopene activate the adiponectin signaling pathway, including AMPK phosphorylation
Abstract
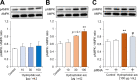
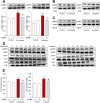
Adiponectin, an adipokine, regulates glucose metabolism and insulin sensitivity through the adiponectin receptor (AdipoR). In this study, we searched for metabolites that activate the adiponectin signaling pathway from tomato (Solanum lycopersicu). Metabolites of mature tomato were separated into 55 fractions by liquid chromatography, and then each fraction was examined using the phosphorylation assay of AMP-protein kinase (AMPK) in C2C12 myotubes and in AdipoR-knockdown cells by small interfering RNA (siRNA). Several fractions showed AMPK phosphorylation in C2C12 myotubes and siRNA-mediated abrogation of the effect. Non-targeted metabolite analysis revealed the presence of 721 diverse metabolites in tomato. By integrating the activity of fractions on AMPK phosphorylation and the 721 metabolites based on their retention times of liquid chromatography, we performed a comprehensive screen for metabolites that possess adiponectin-like activity. As the screening suggested that the active fractions contained four carotenoids, we further analyzed β-carotene and lycopene, the major carotenoids of food. They induced AMPK phosphorylation via the AdipoR, Ca2+/calmodulin-dependent protein kinase kinase and Ca2+ influx, in addition to activating glucose uptake via AdipoR in C2C12 myotubes. All these events were characteristic adiponectin actions. These results indicated that the food-derived carotenoids, β-carotene and lycopene, activate the adiponectin signaling pathway, including AMPK phosphorylation.
Conflict of interest statement
In this study, the authors received funding by KAGOME CO., LTD. This does not alter the authors adherence to PLOS ONE policies on sharing data and materials.
Figures





References
-
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81. doi: 10.1016/S0140-6736(14)60460-8 - DOI - PMC - PubMed
-
- Miranda PJ, DeFronzo RA, Califf RM, Guyton JR. Metabolic syndrome: definition, pathophysiology, and mechanisms. Am Heart J. 2005;149(1):33–45. - PubMed
-
- Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52(7):1779–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

