STAT and Janus kinase targeting by human herpesvirus 8 interferon regulatory factor in the suppression of type-I interferon signaling
- PMID: 35776779
- PMCID: PMC9307175
- DOI: 10.1371/journal.ppat.1010676
STAT and Janus kinase targeting by human herpesvirus 8 interferon regulatory factor in the suppression of type-I interferon signaling
Abstract
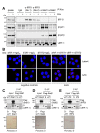
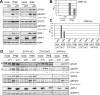
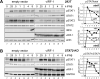
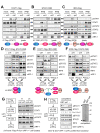
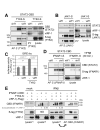
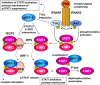
Human herpesvirus 8 (HHV-8), also known as Kaposi's sarcoma (KS)-associated herpesvirus, is involved etiologically in AIDS-associated KS, primary effusion lymphoma (PEL), and multicentric Castleman's disease, in which both viral latent and lytic functions are important. HHV-8 encodes four viral interferon regulatory factors (vIRFs) that are believed to contribute to viral latency (in PEL cells, at least) and/or to productive replication via suppression of cellular antiviral and stress signaling. Here, we identify vIRF-1 interactions with signal transducer and activator of transcription (STAT) factors 1 and 2, interferon (IFN)-stimulated gene factor 3 (ISGF3) cofactor IRF9, and associated signal transducing Janus kinases JAK1 and TYK2. In naturally infected PEL cells and in iSLK epithelial cells infected experimentally with genetically engineered HHV-8, vIRF-1 depletion or ablation, respectively, led to increased levels of active (phosphorylated) STAT1 and STAT2 in IFNβ-treated, and untreated, cells during lytic replication and to associated cellular-gene induction. In transfected 293T cells, used for mechanistic studies, suppression by vIRF-1 of IFNβ-induced phospho-STAT1 (pSTAT1) was found to be highly dependent on STAT2, indicating vIRF-1-mediated inhibition and/or dissociation of ISGF3-complexing, resulting in susceptibility of pSTAT1 to inactivating dephosphorylation. Indeed, coprecipitation experiments involving targeted precipitation of ISGF3 components identified suppression of mutual interactions by vIRF-1. In contrast, suppression of IFNβ-induced pSTAT2 was effected by regulation of STAT2 activation, likely via detected inhibition of TYK2 and its interactions with STAT2 and IFN type-I receptor (IFNAR). Our identified vIRF-1 interactions with IFN-signaling mediators STATs 1 and 2, co-interacting ISGF3 component IRF9, and STAT-activating TYK2 and the suppression of IFN signaling via ISGF3, TYK2-STAT2 and TYK2-IFNAR disruption and TYK2 inhibition represent novel mechanisms of vIRF function and HHV-8 evasion from host-cell defenses.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Human Herpesvirus 8 Interferon Regulatory Factors 1 and 3 Mediate Replication and Latency Activities via Interactions with USP7 Deubiquitinase.J Virol. 2018 Mar 14;92(7):e02003-17. doi: 10.1128/JVI.02003-17. Print 2018 Apr 1. J Virol. 2018. PMID: 29343584 Free PMC article.
-
USP7-Dependent Regulation of TRAF Activation and Signaling by a Viral Interferon Regulatory Factor Homologue.J Virol. 2020 Jan 6;94(2):e01553-19. doi: 10.1128/JVI.01553-19. Print 2020 Jan 6. J Virol. 2020. PMID: 31666375 Free PMC article.
-
Biologically significant interaction of human herpesvirus 8 viral interferon regulatory factor 4 with ubiquitin-specific protease 7.J Virol. 2024 Jun 13;98(6):e0025524. doi: 10.1128/jvi.00255-24. Epub 2024 May 16. J Virol. 2024. PMID: 38752725 Free PMC article.
-
A Positive Feedback Amplifier Circuit That Regulates Interferon (IFN)-Stimulated Gene Expression and Controls Type I and Type II IFN Responses.Front Immunol. 2018 May 28;9:1135. doi: 10.3389/fimmu.2018.01135. eCollection 2018. Front Immunol. 2018. PMID: 29892288 Free PMC article. Review.
-
The unique role of STAT2 in constitutive and IFN-induced transcription and antiviral responses.Cytokine Growth Factor Rev. 2016 Jun;29:71-81. doi: 10.1016/j.cytogfr.2016.02.010. Epub 2016 Mar 18. Cytokine Growth Factor Rev. 2016. PMID: 27053489 Review.
Cited by
-
Polypharmacology-based kinome screen identifies new regulators of KSHV reactivation.PLoS Pathog. 2023 Sep 5;19(9):e1011169. doi: 10.1371/journal.ppat.1011169. eCollection 2023 Sep. PLoS Pathog. 2023. PMID: 37669313 Free PMC article.
-
Polypharmacology-based kinome screen identifies new regulators of KSHV reactivation.bioRxiv [Preprint]. 2023 Feb 1:2023.02.01.526589. doi: 10.1101/2023.02.01.526589. bioRxiv. 2023. Update in: PLoS Pathog. 2023 Sep 5;19(9):e1011169. doi: 10.1371/journal.ppat.1011169. PMID: 36778430 Free PMC article. Updated. Preprint.
-
Molecular Mechanisms of Kaposi Sarcoma-Associated Herpesvirus (HHV8)-Related Lymphomagenesis.Cancers (Basel). 2024 Oct 31;16(21):3693. doi: 10.3390/cancers16213693. Cancers (Basel). 2024. PMID: 39518131 Free PMC article. Review.
-
Direct and biologically significant interactions of human herpesvirus 8 interferon regulatory factor 1 with STAT3 and Janus kinase TYK2.PLoS Pathog. 2023 Nov 20;19(11):e1011806. doi: 10.1371/journal.ppat.1011806. eCollection 2023 Nov. PLoS Pathog. 2023. PMID: 37983265 Free PMC article.
-
Recent updates of interferon-derived myxovirus resistance protein A as a biomarker for acute viral infection.Eur J Med Res. 2024 Dec 23;29(1):612. doi: 10.1186/s40001-024-02221-8. Eur J Med Res. 2024. PMID: 39710743 Free PMC article. Review.
References
-
- Alexander L, Denekamp L, Knapp A, Auerbach MR, Damania B, Desrosiers RC. The primary sequence of rhesus monkey rhadinovirus isolate 26–95: sequence similarities to Kaposi’s sarcoma-associated herpesvirus and rhesus monkey rhadinovirus isolate 17577. J Virol. 2000; 74:3388–98. doi: 10.1128/jvi.74.7.3388-3398.2000 - DOI - PMC - PubMed
-
- Bruce AG, Ryan JT, Thomas MJ, Peng X, Grundhoff A, Tsai CC, et al.. Next-generation sequence analysis of the genome of RFHVMn, the macaque homolog of Kaposi’s sarcoma (KS)-associated herpesvirus, from a KS-like tumor of a pig-tailed macaque. J Virol. 2013; 87:13676–93. doi: 10.1128/JVI.02331-13 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

