Introducing a New Bond-Forming Activity in an Archaeal DNA Polymerase by Structure-Guided Enzyme Redesign
- PMID: 35776893
- PMCID: PMC9442636
- DOI: 10.1021/acschembio.2c00373
Introducing a New Bond-Forming Activity in an Archaeal DNA Polymerase by Structure-Guided Enzyme Redesign
Abstract
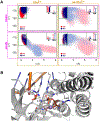
DNA polymerases have evolved to feature a highly conserved activity across the tree of life: formation of, without exception, internucleotidyl O-P linkages. Can this linkage selectivity be overcome by design to produce xenonucleic acids? Here, we report that the structure-guided redesign of an archaeal DNA polymerase, 9°N, exhibits a new activity undetectable in the wild-type enzyme: catalyzing the formation of internucleotidyl N-P linkages using 3'-NH2-ddNTPs. Replacing a metal-binding aspartate in the 9°N active site with asparagine was key to the emergence of this unnatural enzyme activity. MD simulations provided insights into how a single substitution enhances the productive positioning of a 3'-amino nucleophile in the active site. Further remodeling of the protein-nucleic acid interface in the finger subdomain yielded a quadruple-mutant variant (9°N-NRQS) displaying DNA-dependent NP-DNA polymerase activity. In addition, the engineered promiscuity of 9°N-NRQS was leveraged for one-pot synthesis of DNA─NP-DNA copolymers. This work sheds light on the molecular basis of substrate fidelity and latent promiscuity in enzymes.
Figures
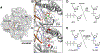




Similar articles
-
Crystal structure of a pol alpha family DNA polymerase from the hyperthermophilic archaeon Thermococcus sp. 9 degrees N-7.J Mol Biol. 2000 Jun 2;299(2):447-62. doi: 10.1006/jmbi.2000.3728. J Mol Biol. 2000. PMID: 10860752
-
Synthesis of phosphoramidate-linked DNA by a modified DNA polymerase.Proc Natl Acad Sci U S A. 2020 Mar 31;117(13):7276-7283. doi: 10.1073/pnas.1922400117. Epub 2020 Mar 18. Proc Natl Acad Sci U S A. 2020. PMID: 32188786 Free PMC article.
-
Insights into eukaryotic primer synthesis from structures of the p48 subunit of human DNA primase.J Mol Biol. 2014 Feb 6;426(3):558-69. doi: 10.1016/j.jmb.2013.11.007. Epub 2013 Nov 13. J Mol Biol. 2014. PMID: 24239947 Free PMC article.
-
The euryarchaeotes, a subdomain of Archaea, survive on a single DNA polymerase: fact or farce?Genes Genet Syst. 1998 Dec;73(6):323-36. doi: 10.1266/ggs.73.323. Genes Genet Syst. 1998. PMID: 10333564 Review.
-
The Extended "Two-Barrel" Polymerases Superfamily: Structure, Function and Evolution.J Mol Biol. 2019 Sep 20;431(20):4167-4183. doi: 10.1016/j.jmb.2019.05.017. Epub 2019 May 17. J Mol Biol. 2019. PMID: 31103775 Review.
Cited by
-
There's more to enzyme antagonism than inhibition.Bioorg Med Chem. 2023 Mar 15;82:117231. doi: 10.1016/j.bmc.2023.117231. Epub 2023 Mar 5. Bioorg Med Chem. 2023. PMID: 36893527 Free PMC article. Review.
-
Towards the controlled enzymatic synthesis of LNA containing oligonucleotides.Front Chem. 2023 Apr 27;11:1161462. doi: 10.3389/fchem.2023.1161462. eCollection 2023. Front Chem. 2023. PMID: 37179777 Free PMC article.
-
Trivalent rare earth metal cofactors confer rapid NP-DNA polymerase activity.Science. 2023 Oct 27;382(6669):423-429. doi: 10.1126/science.adh5339. Epub 2023 Oct 26. Science. 2023. PMID: 37883544 Free PMC article.
References
-
- Rothwell PJ; Waksman G Structure and Mechanism of DNA Polymerases. Adv. Protein Chem 2005, 71, 401–440. - PubMed
-
- Yang W; Lee JY; Nowotny M Making and Breaking Nucleic Acids: Two-Mg2+-Ion Catalysis and Substrate Specificity. Mol. Cell 2006, 22, 5–13. - PubMed
-
- Li Q; Maola VA; Chim N; Hussain J; Lozoya-Colinas A; Chaput JC Synthesis and Polymerase Recognition of Threose Nucleic Acid Triphosphates Equipped with Diverse Chemical Functionalities. J. Am. Chem. Soc 2021, 143, 17761–17768. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

