Genetic testing in severe aplastic anemia is required for optimal hematopoietic cell transplant outcomes
- PMID: 35776903
- PMCID: PMC9412004
- DOI: 10.1182/blood.2022016508
Genetic testing in severe aplastic anemia is required for optimal hematopoietic cell transplant outcomes
Abstract
Patients with severe aplastic anemia (SAA) can have an unrecognized inherited bone marrow failure syndrome (IBMFS) because of phenotypic heterogeneity. We curated germline genetic variants in 104 IBMFS-associated genes from exome sequencing performed on 732 patients who underwent hematopoietic cell transplant (HCT) between 1989 and 2015 for acquired SAA. Patients with pathogenic or likely pathogenic (P/LP) variants fitting known disease zygosity patterns were deemed unrecognized IBMFS. Carriers were defined as patients with a single P/LP variant in an autosomal recessive gene or females with an X-linked recessive P/LP variant. Cox proportional hazard models were used for survival analysis with follow-up until 2017. We identified 113 P/LP single-nucleotide variants or small insertions/deletions and 10 copy number variants across 42 genes in 121 patients. Ninety-one patients had 105 in silico predicted deleterious variants of uncertain significance (dVUS). Forty-eight patients (6.6%) had an unrecognized IBMFS (33% adults), and 73 (10%) were carriers. No survival difference between dVUS and acquired SAA was noted. Compared with acquired SAA (no P/LP variants), patients with unrecognized IBMFS, but not carriers, had worse survival after HCT (IBMFS hazard ratio [HR], 2.13; 95% confidence interval[CI], 1.40-3.24; P = .0004; carriers HR, 0.96; 95% CI, 0.62-1.50; P = .86). Results were similar in analyses restricted to patients receiving reduced-intensity conditioning (n = 448; HR IBMFS = 2.39; P = .01). The excess mortality risk in unrecognized IBMFS attributed to death from organ failure (HR = 4.88; P < .0001). Genetic testing should be part of the diagnostic evaluation for all patients with SAA to tailor therapeutic regimens. Carriers of a pathogenic variant in an IBMFS gene can follow HCT regimens for acquired SAA.
© 2022 by The American Society of Hematology.
Figures


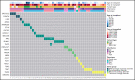
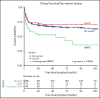
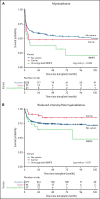
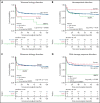
Comment in
-
Playing with fire: unrecognized AA genetic predisposition.Blood. 2022 Aug 25;140(8):805-807. doi: 10.1182/blood.2022017531. Blood. 2022. PMID: 36006677 No abstract available.
References
-
- Scheinberg P, Chen J. Aplastic anemia: what have we learned from animal models and from the clinic. Semin Hematol. 2013;50(2):156-164. - PubMed
-
- Deng XZ, Du M, Peng J, et al. Associations between the HLA-A/B/DRB1 polymorphisms and aplastic anemia: evidence from 17 case-control studies. Hematology. 2018;23(3):154-162. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

