Solid-State NMR Reveals Asymmetric ATP Hydrolysis in the Multidrug ABC Transporter BmrA
- PMID: 35776907
- PMCID: PMC9284561
- DOI: 10.1021/jacs.2c04287
Solid-State NMR Reveals Asymmetric ATP Hydrolysis in the Multidrug ABC Transporter BmrA
Abstract
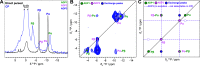
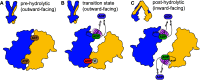
The detailed mechanism of ATP hydrolysis in ATP-binding cassette (ABC) transporters is still not fully understood. Here, we employed 31P solid-state NMR to probe the conformational changes and dynamics during the catalytic cycle by locking the multidrug ABC transporter BmrA in prehydrolytic, transition, and posthydrolytic states, using a combination of mutants and ATP analogues. The 31P spectra reveal that ATP binds strongly in the prehydrolytic state to both ATP-binding sites as inferred from the analysis of the nonhydrolytic E504A mutant. In the transition state of wild-type BmrA, the symmetry of the dimer is broken and only a single site is tightly bound to ADP:Mg2+:vanadate, while the second site is more 'open' allowing exchange with the nucleotides in the solvent. In the posthydrolytic state, weak binding, as characterized by chemical exchange with free ADP and by asymmetric 31P-31P two-dimensional (2D) correlation spectra, is observed for both sites. Revisiting the 13C spectra in light of these findings confirms the conformational nonequivalence of the two nucleotide-binding sites in the transition state. Our results show that following ATP binding, the symmetry of the ATP-binding sites of BmrA is lost in the ATP-hydrolysis step, but is then recovered in the posthydrolytic ADP-bound state.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Dean M.; Rzhetsky A.; Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001, 11, 1156–1166. 10.1101/gr.184901. - DOI - PubMed
- Schneider E.; Hunke S. ATP-binding-cassette (ABC) transport systems: functional and structural aspects of the ATP-hydrolyzing subunits/domains. FEMS Microbiol. Rev. 1998, 22, 1–20. 10.1111/j.1574-6976.1998.tb00358.x. - DOI - PubMed
-
- Kerr I. D. Structure and association of ATP-binding cassette transporter nucleotide-binding domains. Biochim. Biophys. Acta, Biomembr. 2002, 1561, 47–64. 10.1016/S0304-4157(01)00008-9. - DOI - PubMed
- Wilkens S. Structure and mechanism of ABC transporters. F1000Prime Rep. 2015, 7, 14. 10.12703/p7-14. - DOI - PMC - PubMed
- Rees D. C.; Johnson E.; Lewinson O. ABC transporters: the power to change. Nat Rev Mol Cell Biol 2009, 10, 218–227. 10.1038/nrm2646. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

