KIR-favorable TCR-αβ/CD19-depleted haploidentical HCT in children with ALL/AML/MDS: primary analysis of the PTCTC ONC1401 trial
- PMID: 35776909
- PMCID: PMC9918850
- DOI: 10.1182/blood.2022015959
KIR-favorable TCR-αβ/CD19-depleted haploidentical HCT in children with ALL/AML/MDS: primary analysis of the PTCTC ONC1401 trial
Abstract
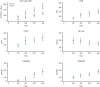
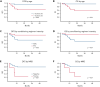
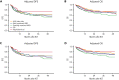
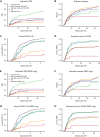
We performed a prospective multicenter study of T-cell receptor αβ (TCR-αβ)/CD19-depleted haploidentical hematopoietic cell transplantation (HCT) in children with acute leukemia and myelodysplastic syndrome (MDS), to determine 1-year disease-free survival (DFS) and compare 2-year outcomes with recipients of other donor cell sources. Fifty-one patients aged 0.7 to 21 years were enrolled; donors were killer immunoglobulin-like receptor (KIR) favorable based on ligand mismatch and/or high B content. The 1-year DFS was 78%. Superior 2-year DFS and overall survival (OS) were noted in patients <10 years of age, those treated with reduced toxicity conditioning (RTC) rather than myeloablative conditioning, and children with minimal residual disease <0.01% before HCT. Multivariate analysis comparing the KIR-favorable haploidentical cohort with controls showed similar DFS and OS compared with other donor cell sources. Multivariate analysis also showed a marked decrease in the risk of grades 2 to 4 and 3 to 4 acute graft versus host disease (aGVHD), chronic GVHD, and transplant-related mortality vs other donor cell sources. Ethnic and racial minorities accounted for 53% of enrolled patients, and data from a large cohort of recipients/donors screened for KIR showed that >80% of recipients had a KIR-favorable donor by our definition, demonstrating that this approach is broadly applicable to groups often unable to find donors. This prospective, multicenter study showed improved outcomes using TCR-αβ/CD19-depleted haploidentical donors using RTC for children with acute leukemia and MDS. Randomized trials comparing this approach with matched unrelated donors are warranted. This trial was registered at https://clinicaltrials.gov as #NCT02646839.
© 2022 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: M.A.P. has served on advisory boards for Novartis, Mesoblast, Equillium, Medexus, and Vertex; has engaged in educational activities for Novartis; and has received study support from Miltenyi (not for this trial) and Adaptive. W.L. is an employee of Miltenyi. C.C.D. has been a consultant to and served on advisory boards of Jazz Pharmaceuticals, Alexion Inc, and Omeros Corporation. J.L.D. receives royalties from Omixon. D.M. is a consultant to, owns stock options in, and receives royalties from Omixon. S.C. has served on advisory boards of AbbVie, Viacord, and Alexion. H.A.-A. received study support from Adaptive. The remaining authors declare no competing financial interests.
Figures

Comment in
-
Believe in haploidentical HSCT: less is better.Blood. 2022 Dec 15;140(24):2523-2524. doi: 10.1182/blood.2022017535. Blood. 2022. PMID: 36520474 No abstract available.
References
-
- Giebel S, Locatelli F, Lamparelli T, et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003;102(3):814–819. - PubMed
-
- Velardi A, Ruggeri L, Mancusi A. Killer-cell immunoglobulin-like receptors reactivity and outcome of stem cell transplant. Curr Opin Hematol. 2012;19(4):319–323. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

