Development and characterization of chlorophyll-amended montmorillonite clays for the adsorption and detoxification of benzene
- PMID: 35777320
- PMCID: PMC9662585
- DOI: 10.1016/j.watres.2022.118788
Development and characterization of chlorophyll-amended montmorillonite clays for the adsorption and detoxification of benzene
Abstract
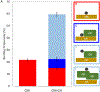
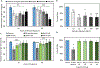
After disasters, such as forest fires and oil spills, high levels of benzene (> 1 ppm) can be detected in the water, soil, and air surrounding the disaster site, which poses a significant health risk to human, animal, and plant populations in the area. While remediation methods with activated carbons have been employed, these strategies are limited in their effectiveness due to benzene's inherent stability and limited retention to most surfaces. To address this problem, calcium and sodium montmorillonite clays were amended with a mixture of chlorophyll (a) and (b); their binding profile and ability to detoxify benzene were characterized using in vitro, in silico, and well-established ecotoxicological (ecotox) bioassay methods. The results of in vitro isothermal analyses indicated that chlorophyll-amended clays showed an improved binding profile in terms of an increased binding affinity (Kf = 668 vs 67), increased binding percentage (52% vs 11%), and decreased rates of desorption (28% vs 100%), compared to the parent clay. In silico simulation studies elucidated the adsorption mechanism and validated that the addition of the chlorophyll to the clays increased the adsorption of benzene through Van der Waals forces (i.e., aromatic π-π stacking and alkyl-π interactions). The sorbents were also assessed for their safety and ability to protect sensitive ecotox organisms (Lemna minor and Caenorhabditis elegans) from the toxicity of benzene. The inclusion of chlorophyll-amended clays in the culture medium significantly reduced benzene toxicity to both organisms, protecting C. elegans by 98-100% from benzene-induced mortality and enhancing the growth rates of L. minor. Isothermal analyses, in silico modeling, and independent bioassays all validated our proof of concept that benzene can be sequestered, tightly bound, and stabilized by chlorophyll-amended montmorillonite clays. These novel sorbents can be utilized during disasters and emergencies to decrease unintentional exposures from contaminated water, soil, and air.
Keywords: Adsorption isotherm; Benzene; Chlorophyll; Molecular simulations; Montmorillonite; Remediation.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Figures
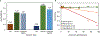
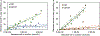
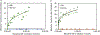
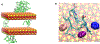


Similar articles
-
Green-Engineered Montmorillonite Clays for the Adsorption, Detoxification, and Mitigation of Aflatoxin B1 Toxicity.Toxins (Basel). 2025 Mar 11;17(3):131. doi: 10.3390/toxins17030131. Toxins (Basel). 2025. PMID: 40137904 Free PMC article.
-
Adsorption of Per- and Polyfluoroalkyl Substances by Edible Nutraceutical-Amended Montmorillonite Clays: In Vitro, In Vivo and In Silico Enterosorption Strategies.Water Air Soil Pollut. 2025;236(5):293. doi: 10.1007/s11270-025-07930-2. Epub 2025 Apr 4. Water Air Soil Pollut. 2025. PMID: 40190788 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Caffeine, riboflavin and curcumin amended clays for PFAS binding.Comput Chem Eng. 2025 Oct;201:109215. doi: 10.1016/j.compchemeng.2025.109215. Epub 2025 May 29. Comput Chem Eng. 2025. PMID: 40852629
Cited by
-
Toxicity of representative organophosphate, organochlorine, phenylurea, dinitroaniline, carbamate, and viologen pesticides to the growth and survival of H. vulgaris, L. minor, and C. elegans.Environ Sci Pollut Res Int. 2024 Mar;31(14):21781-21796. doi: 10.1007/s11356-024-32444-5. Epub 2024 Feb 24. Environ Sci Pollut Res Int. 2024. PMID: 38396181 Free PMC article.
-
β-Lactoglobulin Enhances Clay and Activated Carbon Binding and Protection Properties for Cadmium and Lead.Ind Eng Chem Res. 2024 Sep 6;63(37):16124-16140. doi: 10.1021/acs.iecr.4c01774. eCollection 2024 Sep 18. Ind Eng Chem Res. 2024. PMID: 39319074 Free PMC article.
-
Comparative Analysis of Microalgae's Physiological Responses to Fibrous and Layered Clay Minerals.Biology (Basel). 2025 Jun 3;14(6):647. doi: 10.3390/biology14060647. Biology (Basel). 2025. PMID: 40563898 Free PMC article.
-
Green-Engineered Barrier Creams with Montmorillonite-Chlorophyll Clays as Adsorbents for Benzene, Toluene, and Xylene.Separations. 2023 Apr;10(4):237. doi: 10.3390/separations10040237. Epub 2023 Apr 4. Separations. 2023. PMID: 37251084 Free PMC article.
-
Chlorophyll-Amended Organoclays for the Detoxification of Ochratoxin A.Toxins (Basel). 2024 Nov 6;16(11):479. doi: 10.3390/toxins16110479. Toxins (Basel). 2024. PMID: 39591234 Free PMC article.
References
-
- Aliferis KA, Materzok S, Paziotou GN, Chrysayi-Tokousbalides M, 2009. Lemna minor L. as a model organism for ecotoxicological studies performing 1H NMR fingerprinting. Chemosphere 76 (7), 967–973. - PubMed
-
- Anjum H, Obaid MA, Shamim MY, Murugesan T, 2018. Adsorption of Benzene by “Green” Functionalization of Montmorillonite. EDP Sciences, p. 02001.
-
- ATSDR 2007. Toxicological profile for benzene. - PubMed
-
- Bahrami A, Mahjub H, Sadeghian M, Golbabaei F, 2011. Determination of benzene, toluene and xylene (BTX) concentrations in air using HPLC developed method compared to gas chromatography. Intern. J. Occupat. Hyg 3 (1).
-
- Bennett J, 1981. Biosynthesis of the light-harvesting chlorophyll a/b protein: polypeptide turnover in darkness. Eur. J. Biochem 118 (1), 61–70. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

