Pathogenic analysis of coxsackievirus A10 in rhesus macaques
- PMID: 35777657
- PMCID: PMC9437613
- DOI: 10.1016/j.virs.2022.06.007
Pathogenic analysis of coxsackievirus A10 in rhesus macaques
Abstract
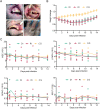
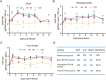
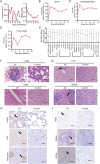
Coxsackievirus A10 (CV-A10) is one of the etiological agents associated with hand, foot and mouth disease (HFMD) and also causes a variety of illnesses in humans, including pneumonia, and myocarditis. Different people, particularly young children, may have different immunological responses to infection. Current CV-A10 infection animal models provide only a rudimentary understanding of the pathogenesis and effects of this virus. The characteristics of CV-A10 infection, replication, and shedding in humans remain unknown. In this study, rhesus macaques were infected by CV-A10 via respiratory or digestive route to mimic the HFMD in humans. The clinical symptoms, viral shedding, inflammatory response and pathologic changes were investigated in acute infection (1-11 day post infection) and recovery period (12-180 day post infection). All infected rhesus macaques during acute infection showed obvious viremia and clinical symptoms which were comparable to those observed in humans. Substantial inflammatory pathological damages were observed in multi-organs, including the lung, heart, liver, and kidney. During the acute period, all rhesus macaques displayed clinical signs, viral shedding, normalization of serum cytokines, and increased serum neutralizing antibodies, whereas inflammatory factors caused some animals to develop severe hyperglycemia during the recovery period. In addition, there were no significant differences between respiratory and digestive tract infected animals. Overall, all data presented suggest that the rhesus macaques provide the first non-human primate animal model for investigating CV-A10 pathophysiology and assessing the development of potential human therapies.
Keywords: Coxsackievirus A10 (CV-A10); Hand, Foot and mouth disease (HFMD); Non-human primate model; Pathogenic analysis; Rhesus macaque.
Copyright © 2022 The Authors. Publishing services by Elsevier B.V. All rights reserved.
Figures

References
-
- Bian L., Gao F., Mao Q., Sun S., Wu X., Liu S., Yang X., Liang Z. Hand, foot, and mouth disease associated with coxsackievirus A10: more serious than it seems. Expert Rev. Anti Infect. Ther. 2019;17:233–242. - PubMed
-
- Chang L., Lin T., Hsu K., Huang Y., Lee C. Clinical features and risk factors of pulmonary oedema after enterovirus-71-related hand, foot, and mouth disease. Lancet. 1999;354:1682–1686. - PubMed
-
- Chang L., Tsao K., Hsia S., Shih S., Huang C., Chan W., Hsu K., Fang T., Huang Y. Transmission and clinical features of enterovirus 71 infections in household contacts in taiwan. JAMA. 2014;291:222–227. - PubMed
-
- Chapman N., Kim K. Persistent coxsackievirus infection: enterovirus persistence in chronic myocarditis and dilated cardiomyopathy. Curr. Top. Microbiol. Immunol. 2008;323:275–292. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

