Non-Invasive Three-Dimensional Cell Analysis in Bioinks by Raman Imaging
- PMID: 35777738
- PMCID: PMC9284518
- DOI: 10.1021/acsami.1c24463
Non-Invasive Three-Dimensional Cell Analysis in Bioinks by Raman Imaging
Abstract
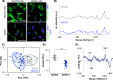
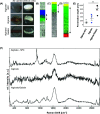
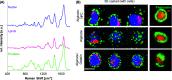
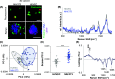
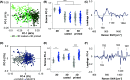
3D bioprinting is an emerging biofabrication strategy using bioinks, comprising cells and biocompatible materials, to produce functional tissue models. Despite progress in building increasingly complex objects, biological analyses in printed constructs remain challenging. Especially, methods that allow non-invasive and non-destructive evaluation of embedded cells are largely missing. Here, we implemented Raman imaging for molecular-sensitive investigations on bioprinted objects. Different aspects such as culture formats (2D, 3D-cast, and 3D-printed), cell types (endothelial cells and fibroblasts), and the selection of the biopolymer (alginate, alginate/nanofibrillated cellulose, alginate/gelatin) were considered and evaluated. Raman imaging allowed for marker-independent identification and localization of subcellular components against the surrounding biomaterial background. Furthermore, single-cell analysis of spectral signatures, performed by multivariate analysis, demonstrated discrimination between endothelial cells and fibroblasts and identified cellular features influenced by the bioprinting process. In summary, Raman imaging was successfully established to analyze cells in 3D culture in situ and evaluate them with regard to the localization of different cell types and their molecular phenotype as a valuable tool for quality control of bioprinted objects.
Keywords: Raman microspectroscopy; bioinks; extrusion-based bioprinting; molecular imaging; non-invasive cell analysis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Sun W.; Starly B.; Daly A. C.; Burdick J. A.; Groll J.; Skeldon G.; Shu W.; Sakai Y.; Shinohara M.; Nishikawa M.; Jang J.; Cho D.-W.; Nie M.; Takeuchi S.; Ostrovidov S.; Khademhosseini A.; Kamm R. D.; Mironov V.; Moroni L.; Ozbolat I. T. The Bioprinting Roadmap. Biofabrication 2020, 12, 022002.10.1088/1758-5090/ab5158. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

