Bone morphogenetic protein 4 inhibits pulmonary fibrosis by modulating cellular senescence and mitophagy in lung fibroblasts
- PMID: 35777761
- PMCID: PMC9808813
- DOI: 10.1183/13993003.02307-2021
Bone morphogenetic protein 4 inhibits pulmonary fibrosis by modulating cellular senescence and mitophagy in lung fibroblasts
Abstract
Background: Accumulation of myofibroblasts is critical to fibrogenesis in idiopathic pulmonary fibrosis (IPF). Senescence and insufficient mitophagy in fibroblasts contribute to their differentiation into myofibroblasts, thereby promoting the development of lung fibrosis. Bone morphogenetic protein 4 (BMP4), a multifunctional growth factor, is essential for the early stage of lung development; however, the role of BMP4 in modulating lung fibrosis remains unknown.
Methods: The aim of this study was to evaluate the role of BMP4 in lung fibrosis using BMP4-haplodeleted mice, BMP4-overexpressed mice, primary lung fibroblasts and lung samples from patients with IPF.
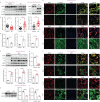
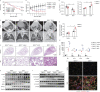
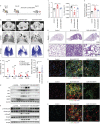
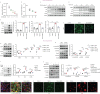
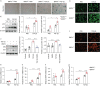
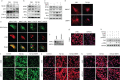
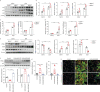
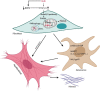
Results: BMP4 expression was downregulated in IPF lungs and fibroblasts compared to control individuals, negatively correlated with fibrotic genes, and BMP4 decreased with transforming growth factor (TGF)-β1 stimulation in lung fibroblasts in a time- and dose-dependent manner. In mice challenged with bleomycin, BMP4 haploinsufficiency perpetuated activation of lung myofibroblasts and caused accelerated lung function decline, severe fibrosis and mortality. BMP4 overexpression using adeno-associated virus 9 vectors showed preventative and therapeutic efficacy against lung fibrosis. In vitro, BMP4 attenuated TGF-β1-induced fibroblast-to-myofibroblast differentiation and extracellular matrix (ECM) production by reducing impaired mitophagy and cellular senescence in lung fibroblasts. Pink1 silencing by short-hairpin RNA transfection abolished the ability of BMP4 to reverse the TGF-β1-induced myofibroblast differentiation and ECM production, indicating dependence on Pink1-mediated mitophagy. Moreover, the inhibitory effect of BMP4 on fibroblast activation and differentiation was accompanied with an activation of Smad1/5/9 signalling and suppression of TGF-β1-mediated Smad2/3 signalling in vivo and in vitro.
Conclusion: Strategies for enhancing BMP4 signalling may represent an effective treatment for pulmonary fibrosis.
Copyright ©The authors 2022.
Conflict of interest statement
Conflict of interest: The authors have nothing to disclose.
Figures
Comment in
-
How a fibroblast ages: a role for bone morphogenetic protein 4 in protecting lung fibroblasts from senescence in pulmonary fibrosis.Eur Respir J. 2022 Dec 15;60(6):2201702. doi: 10.1183/13993003.01702-2022. Print 2022 Dec. Eur Respir J. 2022. PMID: 36522139 Free PMC article. No abstract available.
Similar articles
-
Andrographolide ameliorates bleomycin-induced pulmonary fibrosis by suppressing cell proliferation and myofibroblast differentiation of fibroblasts via the TGF-β1-mediated Smad-dependent and -independent pathways.Toxicol Lett. 2020 Mar 15;321:103-113. doi: 10.1016/j.toxlet.2019.11.003. Epub 2019 Nov 6. Toxicol Lett. 2020. PMID: 31706003
-
Effects of bone morphogenetic protein 4 on TGF-β1-induced cell proliferation, apoptosis, activation and differentiation in mouse lung fibroblasts via ERK/p38 MAPK signaling pathway.PeerJ. 2022 Jul 27;10:e13775. doi: 10.7717/peerj.13775. eCollection 2022. PeerJ. 2022. PMID: 35915750 Free PMC article.
-
Pirfenidone inhibits myofibroblast differentiation and lung fibrosis development during insufficient mitophagy.Respir Res. 2017 Jun 2;18(1):114. doi: 10.1186/s12931-017-0600-3. Respir Res. 2017. PMID: 28577568 Free PMC article.
-
Angiotensin-TGF-beta 1 crosstalk in human idiopathic pulmonary fibrosis: autocrine mechanisms in myofibroblasts and macrophages.Curr Pharm Des. 2007;13(12):1247-56. doi: 10.2174/138161207780618885. Curr Pharm Des. 2007. PMID: 17504233 Review.
-
The Role of Interaction between Mitochondria and the Extracellular Matrix in the Development of Idiopathic Pulmonary Fibrosis.Oxid Med Cell Longev. 2021 Oct 18;2021:9932442. doi: 10.1155/2021/9932442. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34707784 Free PMC article. Review.
Cited by
-
Bone morphogenetic protein 4 thermosensitive hydrogel inhibits corneal neovascularization by repairing corneal epithelial apical junctional complexes.Mater Today Bio. 2024 Jan 4;24:100944. doi: 10.1016/j.mtbio.2024.100944. eCollection 2024 Feb. Mater Today Bio. 2024. PMID: 38269056 Free PMC article.
-
Lung regeneration: diverse cell types and the therapeutic potential.MedComm (2020). 2024 Feb 23;5(2):e494. doi: 10.1002/mco2.494. eCollection 2024 Feb. MedComm (2020). 2024. PMID: 38405059 Free PMC article. Review.
-
Exploring Smad5: a review to pave the way for a deeper understanding of the pathobiology of common respiratory diseases.Mol Med. 2024 Nov 22;30(1):225. doi: 10.1186/s10020-024-00961-1. Mol Med. 2024. PMID: 39578779 Free PMC article. Review.
-
Defining signals that promote human alveolar type I differentiation.Am J Physiol Lung Cell Mol Physiol. 2024 Apr 1;326(4):L409-L418. doi: 10.1152/ajplung.00191.2023. Epub 2024 Feb 13. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 38349124 Free PMC article.
-
Update of Aging Hallmarks in Idiopathic Pulmonary Fibrosis.Cells. 2025 Feb 5;14(3):222. doi: 10.3390/cells14030222. Cells. 2025. PMID: 39937013 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
