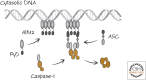
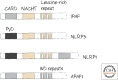
Inflammasomes and Other Caspase-Activation Platforms
- PMID: 35777830
- PMCID: PMC9248825
- DOI: 10.1101/cshperspect.a041061
Inflammasomes and Other Caspase-Activation Platforms
Figures





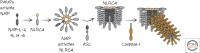
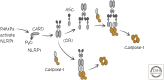

Similar articles
-
Inflammatory Caspases: Toward a Unified Model for Caspase Activation by Inflammasomes.Annu Rev Immunol. 2022 Apr 26;40:249-269. doi: 10.1146/annurev-immunol-101220-030653. Epub 2022 Jan 26. Annu Rev Immunol. 2022. PMID: 35080918 Review.
-
Death and survival from executioner caspase activation.Semin Cell Dev Biol. 2024 Mar 15;156:66-73. doi: 10.1016/j.semcdb.2023.07.005. Epub 2023 Jul 18. Semin Cell Dev Biol. 2024. PMID: 37468421 Review.
-
Inflammasomes as polyvalent cell death platforms.Cell Mol Life Sci. 2016 Jun;73(11-12):2335-47. doi: 10.1007/s00018-016-2204-3. Epub 2016 Apr 5. Cell Mol Life Sci. 2016. PMID: 27048821 Free PMC article. Review.
-
Discovery of a caspase cleavage motif antibody reveals insights into noncanonical inflammasome function.Proc Natl Acad Sci U S A. 2021 Mar 23;118(12):e2018024118. doi: 10.1073/pnas.2018024118. Proc Natl Acad Sci U S A. 2021. PMID: 33723046 Free PMC article.
-
Caspase-11: the driving factor for noncanonical inflammasomes.Eur J Immunol. 2013 Sep;43(9):2240-5. doi: 10.1002/eji.201343800. Eur J Immunol. 2013. PMID: 24037676 Review.
Cited by
-
Caspase-2 kills cells with extra centrosomes.Sci Adv. 2024 Nov;10(44):eado6607. doi: 10.1126/sciadv.ado6607. Epub 2024 Oct 30. Sci Adv. 2024. PMID: 39475598 Free PMC article.
-
The Future of Death.Cold Spring Harb Perspect Biol. 2022 Dec 1;14(12):a041111. doi: 10.1101/cshperspect.a041111. Cold Spring Harb Perspect Biol. 2022. PMID: 36456104 Free PMC article. Review. No abstract available.
-
Caspase-5: Structure, Pro-Inflammatory Activity and Evolution.Biomolecules. 2024 Apr 26;14(5):520. doi: 10.3390/biom14050520. Biomolecules. 2024. PMID: 38785927 Free PMC article. Review.
-
The Burial: Clearance and Consequences.Cold Spring Harb Perspect Biol. 2022 Oct 3;14(10):a041087. doi: 10.1101/cshperspect.a041087. Cold Spring Harb Perspect Biol. 2022. PMID: 36192119 Free PMC article. Review. No abstract available.
-
Stepwise phosphorylation and SUMOylation of PIDD1 drive PIDDosome assembly in response to DNA repair failure.Nat Commun. 2024 Oct 24;15(1):9195. doi: 10.1038/s41467-024-53412-0. Nat Commun. 2024. PMID: 39448602 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
