Protective effects of omega-3 fatty acids in a blood-brain barrier-on-chip model and on postoperative delirium-like behaviour in mice
- PMID: 35778276
- PMCID: PMC9997088
- DOI: 10.1016/j.bja.2022.05.025
Protective effects of omega-3 fatty acids in a blood-brain barrier-on-chip model and on postoperative delirium-like behaviour in mice
Abstract
Background: Peripheral surgical trauma can trigger neuroinflammation and ensuing neurological complications, such as delirium. The mechanisms whereby surgery contributes to postoperative neuroinflammation remain unclear and without effective therapies. Here, we developed a microfluidic-assisted blood-brain barrier (BBB) device and tested the effects of omega-3 fatty acids on neuroimmune interactions after orthopaedic surgery.
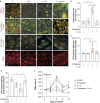
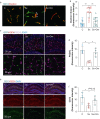
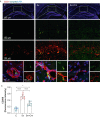
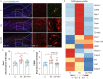
Methods: A microfluidic-assisted BBB device was established using primary human cells. Tight junction proteins, vascular cell adhesion molecule 1 (VCAM-1), BBB permeability, and astrocytic networks were assessed after stimulation with interleukin (IL)-1β and in the presence or absence of a clinically available omega-3 fatty acid emulsion (Omegaven®; Fresenius Kabi, Bad Homburg, Germany). Mice were treated 1 h before orthopaedic surgery with 10 μl g-1 body weight of omega-3 fatty acid emulsion i.v. or equal volumes of saline. Changes in pericytes, perivascular macrophages, BBB opening, microglial activation, and inattention were evaluated.
Results: Omega-3 fatty acids protected barrier permeability, endothelial tight junctions, and VCAM-1 after exposure to IL-1β in the BBB model. In vivo studies confirmed that omega-3 fatty acid treatment inhibited surgery-induced BBB impairment, microglial activation, and delirium-like behaviour. We identified a novel role for pericyte loss and perivascular macrophage activation in mice after surgery, which were rescued by prophylaxis with i.v. omega-3 fatty acids.
Conclusions: We present a new approach to study neuroimmune interactions relevant to perioperative recovery using a microphysiological BBB platform. Changes in barrier function, including dysregulation of pericytes and perivascular macrophages, provide new targets to reduce postoperative delirium.
Keywords: blood–brain barrier; delirium; microglia; neurovascular unit; omega-3 fatty acid; organ-on-chip; pericyte; perivascular macrophage.
Copyright © 2022 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures

Comment on
-
Microfluidics-assisted blood-brain barrier device: a powerful tool to study perioperative neurocognitive disorder.Br J Anaesth. 2023 Feb;130(2):e212-e214. doi: 10.1016/j.bja.2022.08.024. Epub 2022 Sep 29. Br J Anaesth. 2023. PMID: 36182556
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

