Detection of SARS-CoV-2 in exhaled breath from non-hospitalized COVID-19-infected individuals
- PMID: 35778461
- PMCID: PMC9247943
- DOI: 10.1038/s41598-022-15243-1
Detection of SARS-CoV-2 in exhaled breath from non-hospitalized COVID-19-infected individuals
Abstract
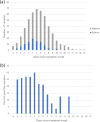
The diagnosis of COVID-19 is based on detection of SARS-CoV-2 in oro-/nasopharyngel swabs, but due to discomfort and minor risk during the swab procedure, detection of SARS-CoV-2 has been investigated in other biological matrixes. In this proof-of-concept study, individuals with confirmed SARS-CoV-2 infection performed a daily air sample for five days. Air samples were obtained through a non-invasive electrostatic air sampler. Detection of SARS-CoV-2 RNA was determined with qRT-PCR. The association of positive samples with different exposures was evaluated through mixed-effect models. We obtained 665 air samples from 111 included participants with confirmed SARS-CoV-2 infection. Overall, 52 individuals (46.8%) had at least one positive air sample, and 129 (19.4%) air samples were positive for SARS-CoV-2. Participants with symptoms or a symptom duration ≤ four days had significantly higher odds of having a positive air sample. Cycle threshold values were significantly lower in samples obtained ≤ 4 days from symptom onset. Neither variant of SARS-CoV-2 nor method of air sampling were associated with a positive air sample. We demonstrate that SARS-CoV-2 is detectable in human breath by electrostatic air sampling with the highest detection rate closest to symptom onset. We suggest further evaluation of the air sampling technique to increase sensitivity.
© 2022. The Author(s).
Conflict of interest statement
J.S. reports employment of AeroCollect A/S, Brøndby, Denmark. K.U. and L.K.D. report employment of FORCE Technology, Brøndby, Denmark. All other authors report no potential conflicts.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

