Extracellular vesicles deliver sodium iodide symporter protein and promote cancer cell radioiodine therapy
- PMID: 35778503
- PMCID: PMC9249836
- DOI: 10.1038/s41598-022-15524-9
Extracellular vesicles deliver sodium iodide symporter protein and promote cancer cell radioiodine therapy
Abstract
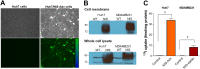
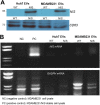
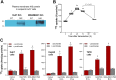
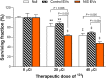
Extracellular vesicles (EVs) are a promising carrier for various cargos with antitumor effects, but their capacity to transfer the ability to transport radioiodine for cancer theranostics remains unexplored. Herein, we tested the hypothesis that EVs can be loaded with the sodium iodide symporter (NIS) protein and efficiently deliver the payload to recipient cancer cells to facilitate radioiodine uptake. The results revealed that donor cells either transduced with an adenoviral vector for transient expression or engineered for stable overexpression secreted EVs that contained substantial amounts of NIS protein but not NIS mRNA. Huh7 liver cancer cells treated with EVs secreted from each of the donor cell types showed significantly increased plasma membrane NIS protein, indicating efficient payload delivery. Furthermore, intact function of the delivered NIS protein was confirmed by significantly increased radioiodine transport in recipient cancer cells that peaked at 48 h. Importantly, NIS protein delivered by EVs significantly enhanced the antitumor effects of 131I radiotherapy. These results reveal that EVs are a promising vehicle to deliver NIS protein to cancer cells in sufficient amounts for radioiodine-based theranostics.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
A novel strategy of transferring NIS protein to cells using extracellular vesicles leads to increase in iodine uptake and cytotoxicity.Int J Nanomedicine. 2019 Mar 7;14:1779-1787. doi: 10.2147/IJN.S189738. eCollection 2019. Int J Nanomedicine. 2019. PMID: 30880979 Free PMC article.
-
Targeting of tumor radioiodine therapy by expression of the sodium iodide symporter under control of the survivin promoter.Cancer Gene Ther. 2011 Feb;18(2):144-52. doi: 10.1038/cgt.2010.66. Epub 2010 Oct 29. Cancer Gene Ther. 2011. PMID: 21037556 Free PMC article.
-
Sodium/iodide symporter: a key transport system in thyroid cancer cell metabolism.Eur J Endocrinol. 1999 Nov;141(5):443-57. doi: 10.1530/eje.0.1410443. Eur J Endocrinol. 1999. PMID: 10576759 Review.
-
Rat sodium iodide symporter allows using lower dose of 131I for cancer therapy.Gene Ther. 2006 Jul;13(13):1052-6. doi: 10.1038/sj.gt.3302758. Epub 2006 Mar 9. Gene Ther. 2006. PMID: 16525480
-
Sodium iodide symporter: its role in nuclear medicine.J Nucl Med. 2002 Sep;43(9):1188-200. J Nucl Med. 2002. PMID: 12215558 Review.
Cited by
-
Engineered exosomes in emerging cell-free therapy.Front Oncol. 2024 Mar 26;14:1382398. doi: 10.3389/fonc.2024.1382398. eCollection 2024. Front Oncol. 2024. PMID: 38595822 Free PMC article. Review.
-
Engineered exosomes from different sources for cancer-targeted therapy.Signal Transduct Target Ther. 2023 Mar 15;8(1):124. doi: 10.1038/s41392-023-01382-y. Signal Transduct Target Ther. 2023. PMID: 36922504 Free PMC article. Review.
-
Role of Extracellular Vesicles in Thyroid Physiology and Diseases: Implications for Diagnosis and Treatment.Biomedicines. 2022 Oct 15;10(10):2585. doi: 10.3390/biomedicines10102585. Biomedicines. 2022. PMID: 36289847 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

