Differential epithelial and stromal LGR5 expression in ovarian carcinogenesis
- PMID: 35778589
- PMCID: PMC9249864
- DOI: 10.1038/s41598-022-15234-2
Differential epithelial and stromal LGR5 expression in ovarian carcinogenesis
Abstract
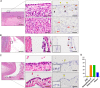
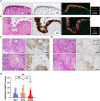
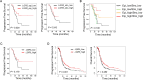
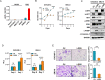
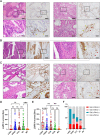
Lgr5 has been identified as a marker of the stem/progenitor cells in the murine ovary and oviduct by lineage tracing. However, little is known regarding LGR5 expression or its functional significance in human ovary tissues. Here, using RNA in situ hybridization and/or immunohistochemistry, we thoroughly investigated LGR5 expression in normal human ovaries, fallopian tubes and various ovarian tumors. We discovered that LGR5 expression is negligible in the human ovary surface epithelium, whereas ovarian stromal cells normally express low levels of LGR5. Remarkably, fallopian tube epithelium, inclusion cysts and serous cystadenomas with a Müllerian phenotype expressed high levels of LGR5, and LGR5 expression was restricted to PAX8+/FOXJ1- secretory cells of the tubal epithelium. Strong stromal LGR5 expression without epithelial LGR5 expression was consistently observed in the path from serous cystadenoma to serous borderline tumor to low grade serous carcinoma (LGSC). Unlike LGSC, high grade serous carcinoma (HGSC), clear cell carcinoma, endometrioid carcinomas displayed various epithelial-stromal LGR5 expression. Notably, high levels of LGR5 expression were observed in serous tubal intraepithelial carcinoma, which slightly declined in invasive HGSC. LGR5 expression was significantly associated with improved progression-free survival in HGSC patients. Moreover, in vitro assays demonstrated that LGR5 expression suppressed tumor proliferation and migratory capabilities. Taken together, these findings indicate a tumor-suppressive role for LGR5 in the progression of HGSC.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA 71, 209–249 (2021). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

