Guanabenz ameliorates disease in vanishing white matter mice in contrast to sephin1
- PMID: 35778832
- PMCID: PMC9380178
- DOI: 10.1002/acn3.51611
Guanabenz ameliorates disease in vanishing white matter mice in contrast to sephin1
Abstract
Objective: Vanishing white matter (VWM) is a leukodystrophy, characterized by stress-sensitive neurological deterioration and premature death. It is currently without curative treatment. It is caused by bi-allelic pathogenic variants in the genes encoding eukaryotic initiation factor 2B (eIF2B). eIF2B is essential for the regulation of the integrated stress response (ISR), a physiological response to cellular stress. Preclinical studies on VWM mouse models revealed that deregulated ISR is key in the pathophysiology of VWM and an effective treatment target. Guanabenz, an α2-adrenergic agonist, attenuates the ISR and has beneficial effects on VWM neuropathology. The current study aimed at elucidating guanabenz's disease-modifying potential and mechanism of action in VWM mice. Sephin1, an ISR-modulating guanabenz analog without α2-adrenergic agonistic properties, was included to separate effects on the ISR from α2-adrenergic effects.
Methods: Wild-type and VWM mice were subjected to placebo, guanabenz or sephin1 treatments. Effects on clinical signs, neuropathology, and ISR deregulation were determined. Guanabenz's and sephin1's ISR-modifying effects were tested in cultured cells that expressed or lacked the α2-adrenergic receptor.
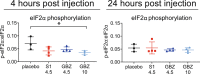
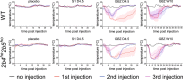
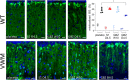
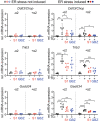
Results: Guanabenz improved clinical signs, neuropathological hallmarks, and ISR regulation in VWM mice, but sephin1 did not. Guanabenz's effects on the ISR in VWM mice were not replicated in cell cultures and the contribution of α2-adrenergic effects on the deregulated ISR could therefore not be assessed.
Interpretation: Guanabenz proved itself as a viable treatment option for VWM. The exact mechanism through which guanabenz exerts its ameliorating impact on VWM requires further studies. Sephin1 is not simply a guanabenz replacement without α2-adrenergic effects.
© 2022 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
MSvdK and TEMA have a patent PCT/NL2018/050293 on guanabenz in VWM pending to VUmc. Otherwise, the authors have declared no competing interest exists.
Figures





Similar articles
-
Pridopidine subtly ameliorates motor skills in a mouse model for vanishing white matter.Life Sci Alliance. 2024 Jan 3;7(3):e202302199. doi: 10.26508/lsa.202302199. Print 2024 Mar. Life Sci Alliance. 2024. PMID: 38171595 Free PMC article.
-
Vanishing white matter: deregulated integrated stress response as therapy target.Ann Clin Transl Neurol. 2019 Aug;6(8):1407-1422. doi: 10.1002/acn3.50826. Epub 2019 Jul 18. Ann Clin Transl Neurol. 2019. PMID: 31402619 Free PMC article.
-
Isocaloric low protein diet in a mouse model for vanishing white matter does not impact ISR deregulation in brain, but reveals ISR deregulation in liver.Nutr Neurosci. 2022 Jun;25(6):1219-1230. doi: 10.1080/1028415X.2020.1846356. Epub 2020 Nov 25. Nutr Neurosci. 2022. PMID: 33236691
-
Vanishing white matter.Handb Clin Neurol. 2024;204:77-94. doi: 10.1016/B978-0-323-99209-1.00015-6. Handb Clin Neurol. 2024. PMID: 39322396 Review.
-
[Eukaryotic translation initiation factor 2B and leukoencephalopathy with vanishing white matter].Beijing Da Xue Xue Bao Yi Xue Ban. 2009 Oct 18;41(5):608-10. Beijing Da Xue Xue Bao Yi Xue Ban. 2009. PMID: 19829687 Review. Chinese.
Cited by
-
In vivo base editing of a pathogenic Eif2b5 variant improves vanishing white matter phenotypes in mice.Mol Ther. 2024 May 1;32(5):1328-1343. doi: 10.1016/j.ymthe.2024.03.009. Epub 2024 Mar 7. Mol Ther. 2024. PMID: 38454603 Free PMC article.
-
An atypical initial revelation of CACH-vanishing white matter syndrome miming herpetic encephalitis in a 6-year-old child: Case report and brief review.Radiol Case Rep. 2025 Apr 10;20(6):3148-3152. doi: 10.1016/j.radcr.2025.02.023. eCollection 2025 Jun. Radiol Case Rep. 2025. PMID: 40247960 Free PMC article.
-
Mammalian integrated stress responses in stressed organelles and their functions.Acta Pharmacol Sin. 2024 Jun;45(6):1095-1114. doi: 10.1038/s41401-023-01225-0. Epub 2024 Jan 24. Acta Pharmacol Sin. 2024. PMID: 38267546 Free PMC article. Review.
-
Core protocol development for phase 2/3 clinical trials in the leukodystrophy vanishing white matter: a consensus statement by the VWM consortium and patient advocates.BMC Neurol. 2023 Aug 17;23(1):305. doi: 10.1186/s12883-023-03354-9. BMC Neurol. 2023. PMID: 37592248 Free PMC article.
-
Secretomics Alterations and Astrocyte Dysfunction in Human iPSC of Leukoencephalopathy with Vanishing White Matter.Neurochem Res. 2022 Dec;47(12):3747-3760. doi: 10.1007/s11064-022-03765-z. Epub 2022 Oct 5. Neurochem Res. 2022. PMID: 36198922
References
-
- Dooves S, Bugiani M, Wisse LE, Abbink TEM, van der Knaap MS, Heine VM. Bergmann glia translocation: a new disease marker for vanishing white matter identifies therapeutic effects of Guanabenz treatment. Neuropathol Appl Neurobiol. 2018;44:391‐403. - PubMed
-
- van der Knaap MS, Leegwater PA, Konst AA, et al. Mutations in each of the five subunits of translation initiation factor eIF2B can cause leukoencephalopathy with vanishing white matter. Ann Neurol. 2002;51:264‐270. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
