Interplay between traveling wave propagation and amplification at the apex of the mouse cochlea
- PMID: 35778839
- PMCID: PMC9388393
- DOI: 10.1016/j.bpj.2022.06.029
Interplay between traveling wave propagation and amplification at the apex of the mouse cochlea
Abstract
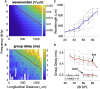
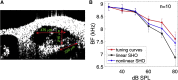
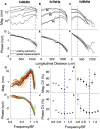
Sounds entering the mammalian ear produce waves that travel from the base to the apex of the cochlea. An electromechanical active process amplifies traveling wave motions and enables sound processing over a broad range of frequencies and intensities. The cochlear amplifier requires combining the global traveling wave with the local cellular processes that change along the length of the cochlea given the gradual changes in hair cell and supporting cell anatomy and physiology. Thus, we measured basilar membrane (BM) traveling waves in vivo along the apical turn of the mouse cochlea using volumetric optical coherence tomography and vibrometry. We found that there was a gradual reduction in key features of the active process toward the apex. For example, the gain decreased from 23 to 19 dB and tuning sharpness decreased from 2.5 to 1.4. Furthermore, we measured the frequency and intensity dependence of traveling wave properties. The phase velocity was larger than the group velocity, and both quantities gradually decrease from the base to the apex denoting a strong dispersion characteristic near the helicotrema. Moreover, we found that the spatial wavelength along the BM was highly level dependent in vivo, such that increasing the sound intensity from 30 to 90 dB sound pressure level increased the wavelength from 504 to 874 μm, a factor of 1.73. We hypothesize that this wavelength variation with sound intensity gives rise to an increase of the fluid-loaded mass on the BM and tunes its local resonance frequency. Together, these data demonstrate a strong interplay between the traveling wave propagation and amplification along the length of the cochlea.
Copyright © 2022 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




Similar articles
-
Noninvasive in vivo imaging reveals differences between tectorial membrane and basilar membrane traveling waves in the mouse cochlea.Proc Natl Acad Sci U S A. 2015 Mar 10;112(10):3128-33. doi: 10.1073/pnas.1500038112. Epub 2015 Mar 3. Proc Natl Acad Sci U S A. 2015. PMID: 25737536 Free PMC article.
-
The physics of hearing: fluid mechanics and the active process of the inner ear.Rep Prog Phys. 2014 Jul;77(7):076601. doi: 10.1088/0034-4885/77/7/076601. Epub 2014 Jul 9. Rep Prog Phys. 2014. PMID: 25006839 Review.
-
Longitudinal pattern of basilar membrane vibration in the sensitive cochlea.Proc Natl Acad Sci U S A. 2002 Dec 24;99(26):17101-6. doi: 10.1073/pnas.262663699. Epub 2002 Dec 2. Proc Natl Acad Sci U S A. 2002. PMID: 12461165 Free PMC article.
-
Mechanical tuning and amplification within the apex of the guinea pig cochlea.J Physiol. 2017 Jul 1;595(13):4549-4561. doi: 10.1113/JP273881. Epub 2017 May 21. J Physiol. 2017. PMID: 28382742 Free PMC article.
-
Mechanics of the mammalian cochlea.Physiol Rev. 2001 Jul;81(3):1305-52. doi: 10.1152/physrev.2001.81.3.1305. Physiol Rev. 2001. PMID: 11427697 Free PMC article. Review.
Cited by
-
Intracochlear overdrive: Characterizing nonlinear wave amplification in the mouse apex.J Acoust Soc Am. 2023 Nov 1;154(5):3414-3428. doi: 10.1121/10.0022446. J Acoust Soc Am. 2023. PMID: 38015028 Free PMC article.
-
Otoacoustic emissions reveal the micromechanical role of organ-of-Corti cytoarchitecture in cochlear amplification.Proc Natl Acad Sci U S A. 2023 Oct 10;120(41):e2305921120. doi: 10.1073/pnas.2305921120. Epub 2023 Oct 5. Proc Natl Acad Sci U S A. 2023. PMID: 37796989 Free PMC article.
-
Cochlear Amplification in the Short-Wave Region by Outer Hair Cells changing Organ-of-Corti area to Amplify the Fluid Traveling Wave.Hear Res. 2022 Dec;426:108641. doi: 10.1016/j.heares.2022.108641. Epub 2022 Oct 21. Hear Res. 2022. PMID: 39776694 Free PMC article.
-
The Medial Olivocochlear Efferent Pathway Potentiates Cochlear Amplification in Response to Hearing Loss.J Neurosci. 2025 Apr 9;45(15):e2103242025. doi: 10.1523/JNEUROSCI.2103-24.2025. J Neurosci. 2025. PMID: 39984203
-
The Reduced Cortilymph Flow Path in the Short-Wave Region Allows Outer Hair Cells to Produce Focused Traveling-Wave Amplification.J Assoc Res Otolaryngol. 2025 Feb;26(1):49-61. doi: 10.1007/s10162-025-00976-3. Epub 2025 Feb 7. J Assoc Res Otolaryngol. 2025. PMID: 39920422
References
-
- V Bekesy G. McGraw Hill; New York: 1960. Experiments in Hearing.
-
- Zwislocki J. Review of recent mathematical theories of cochlear dynamics. J. Acoust. Soc. Am. 1953;25:743–751. doi: 10.1121/1.1907170. - DOI
-
- Helmholtz H.L.F. Cambridge University Press; 1912. On the Sensations of Tone as a Physiological Basis for the Theory of Music.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

