Structurally-engineered fatty acid 1024 (SEFA-1024) improves diet-induced obesity, insulin resistance, and fatty liver disease
- PMID: 35778847
- PMCID: PMC9463121
- DOI: 10.1002/lipd.12351
Structurally-engineered fatty acid 1024 (SEFA-1024) improves diet-induced obesity, insulin resistance, and fatty liver disease
Abstract
Obesity is a global epidemic that drives morbidity and mortality through cardiovascular disease, diabetes, and non-alcoholic fatty liver disease (NAFLD). No definitive therapy has been approved to improve glycemic control and treat NAFLD in obese patients. Here, we investigated a semi-synthetic, long chain, structurally-engineered fatty acid-1024 (SEFA-1024), as a treatment for obesity-induced hyperglycemia, insulin-resistance, and fatty liver disease in rodent models. A single dose of SEFA-1024 was administered to evaluate glucose tolerance and active glucagon-like peptide 1 (GLP-1) in lean rats in the presence and absence of a DPP-4 inhibitor. The effects of SEFA-1024 on weight loss and glycemic control were assessed in genetic (ob/ob) and environmental (high-fat diet) murine models of obesity. Liver histology, serum liver enzymes, liver lipidomics, and hepatic gene expression were also assessed in the high-fat diet murine model. SEFA-1024 reversed obesity-associated insulin resistance and improved glycemic control. SEFA-1024 increased active GLP-1. In a long-term model of diet-induced obesity, SEFA-1024 reversed excessive weight gain, hepatic steatosis, elevated liver enzymes, hepatic lipotoxicity, and promoted fatty acid metabolism. SEFA-1024 is an enterohepatic-targeted, eicosapentaenoic acid derivative that reverses obesity-induced dysregulated glucose metabolism and hepatic lipotoxicity in genetic and dietary rodent models of obesity. The mechanism by which SEFA-1024 works may include increasing aGLP-1, promoting fatty acid oxidation, and inhibiting hepatic triglyceride formation. SEFA-1024 may serve as a potential treatment for obesity-related diabetes and NAFLD.
Keywords: fatty acid metabolism; free fatty acid receptors (FFARs); high-fat diet (HFD); hyperglycemia; hyperinsulinemia; lipidomics; lipotoxicity; obesity; structurally-engineered fatty acid (SEFA).
© 2022 AOCS.
Conflict of interest statement
CONFLICT OF INTEREST
K Gura is a consultant for Pronova/BASF, NorthSea Therapeutics, Xellia Pharmaceuticals, Pfizer Pediatric Center of Excellence, Baxter, and has received research support from NorthSea Therapeutics, Otsuka Pharmaceutical Company, Alcresta, and Fresenius Kabi. M Puder is a consultant for Pronova/BASF, NorthSea Therapeutics, and has received research support from NorthSea Therapeutics, Otsuka Pharmaceutical Company, Alcresta, and Fresenius Kabi; Patent/Royalties for Omegaven are forthcoming. D Fraser is the chief scientific officer for NorthSea Therapeutics, the company that owns the intellectual property to SEFA-1024. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants, or patents received or pending, or royalties.
Figures
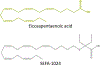





Similar articles
-
A medium-chain fatty acid analogue prevents hepatosteatosis and decreases inflammatory lipid metabolites in a murine model of parenteral nutrition-induced hepatosteatosis.PLoS One. 2023 Dec 1;18(12):e0295244. doi: 10.1371/journal.pone.0295244. eCollection 2023. PLoS One. 2023. PMID: 38039287 Free PMC article.
-
Characteristics of bile acid composition in high fat diet-induced nonalcoholic fatty liver disease in obese diabetic rats.PLoS One. 2021 Feb 24;16(2):e0247303. doi: 10.1371/journal.pone.0247303. eCollection 2021. PLoS One. 2021. PMID: 33626072 Free PMC article.
-
Ugonin J improves metabolic disorder and ameliorates nonalcoholic fatty liver disease by regulating the AMPK/AKT signaling pathway.Pharmacol Res. 2021 Jan;163:105298. doi: 10.1016/j.phrs.2020.105298. Epub 2020 Nov 18. Pharmacol Res. 2021. PMID: 33220422
-
Potential roles of glucagon-like peptide-1-based therapies in treating non-alcoholic fatty liver disease.World J Gastroenterol. 2014 Jul 21;20(27):9090-7. doi: 10.3748/wjg.v20.i27.9090. World J Gastroenterol. 2014. PMID: 25083081 Free PMC article. Review.
-
Olive Oil's Attenuating Effects on Lipotoxicity.Adv Exp Med Biol. 2024;1460:869-882. doi: 10.1007/978-3-031-63657-8_29. Adv Exp Med Biol. 2024. PMID: 39287875 Review.
Cited by
-
Association Between Alcohol Consumption, Other Healthy Habits and Sociodemographic Variables and the Values of Different Insulin Resistance Risk Scales in 139,634 Spanish Workers.Healthcare (Basel). 2025 Apr 17;13(8):921. doi: 10.3390/healthcare13080921. Healthcare (Basel). 2025. PMID: 40281870 Free PMC article.
-
The Related Metabolic Diseases and Treatments of Obesity.Healthcare (Basel). 2022 Aug 25;10(9):1616. doi: 10.3390/healthcare10091616. Healthcare (Basel). 2022. PMID: 36141228 Free PMC article. Review.
-
Chewing the fat: How lipidomics is changing our understanding of human health and disease in 2022.Anal Sci Adv. 2023 May 10;4(3-4):104-131. doi: 10.1002/ansa.202300009. eCollection 2023 May. Anal Sci Adv. 2023. PMID: 38715925 Free PMC article. Review.
References
-
- Adams LA, Waters OR, Knuiman MW, Elliott RR, Olynyk JK. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: an eleven-year follow-up study. Am J Gastroenterol. 2009;104(4):861–7. - PubMed
-
- Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie T. Annual deaths attributable to obesity in the United States. JAMA. 1999; 282(16):1530–8. - PubMed
-
- Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. 2019;16(7):411–28. - PubMed
-
- Barr J, Vázquez-Chantada M, Alonso C, Pérez-Cormenzana M, Mayo R, Galán A, et al. Liquid chromatography-mass spectrometry-based parallel metabolic profiling of human and mouse model serum reveals putative biomarkers associated with the progression of nonalcoholic fatty liver disease. J Proteome Res. 2010;9(9):4501–12. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

