Patient reporting of sexual adverse events on an online platform for medication experiences
- PMID: 35778921
- PMCID: PMC9796902
- DOI: 10.1111/bcp.15454
Patient reporting of sexual adverse events on an online platform for medication experiences
Abstract
Aims: For >300 drugs, sexual side effects are included in the drug information leaflet. As sexual adverse events (sAEs) may be more easily shared at online medication platforms, patient-reported drug experiences may add to the current knowledge on sAE experiences. This study evaluated patient reports from the online platform mijnmedicijn.nl for the frequency of sAE reporting, sex differences concerning sAEs and to assess drugs with disproportional sAE reporting.
Methods: On the online platform, terms for sAEs as used by patients were collected with a poll. Subsequently, drug reports posted between 2008 and 2020 were searched for sAEs with the identified terms. From the retrieved reports, the sAE frequencies and complaints and reporting odds ratios (ROR) were calculated, stratified for sex and drug (class). sAE reporting was considered disproportional frequent if the lower 95% confidence interval bound of the ROR >2.0.
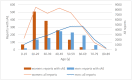
Results: For 189 drugs, sAEs were identified in 2408 reports (3.9%). Women posted 1383 reports (3.5% of all female reports) and men 1025 (4.7%). Almost half of the sAE reports addressed antidepressants: 586 reports of women (ROR 4.2; 95%CI 3.8-4.7) and 510 reports of men (ROR 7.5; 95%CI 6.6-8.5). Disproportional high numbers of sAE reports were found for 27 drugs, mostly antidepressants, hormonal contraceptives and drugs used in benign prostatic hyperplasia. Of these drugs with frequent sAEs, 7 had low sAE risks in their professional drug information.
Conclusion: One in 25 drug reports on mijnmedicijn.nl included sAEs. The sAEs were reported frequently for antidepressants, contraceptives and drugs used in benign prostatic hyperplasia.
Keywords: patient-reported outcome measures-sex differences; pharmacovigilance; sexual adverse events; sexual dysfunction-adverse drug reaction reporting.
© 2022 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
W.W. is the owner and chief executive director of Insight Pharma Services. E.K. was an employee of Insight Pharmacy Services. The remaining authors have no conflicts of interest to declare.
Figures
Similar articles
-
Drug-Associated Acute Kidney Injury Identified in the United States Food and Drug Administration Adverse Event Reporting System Database.Pharmacotherapy. 2018 Aug;38(8):785-793. doi: 10.1002/phar.2152. Epub 2018 Jul 13. Pharmacotherapy. 2018. PMID: 29883524
-
Comparison of serious adverse events posted at ClinicalTrials.gov and published in corresponding journal articles.BMC Med. 2015 Aug 14;13:189. doi: 10.1186/s12916-015-0430-4. BMC Med. 2015. PMID: 26269118 Free PMC article.
-
Reports of sexual disorders related to serotonin reuptake inhibitors in the French pharmacovigilance database: an example of underreporting.Drug Saf. 2013 Jul;36(7):515-9. doi: 10.1007/s40264-013-0069-z. Drug Saf. 2013. PMID: 23728950
-
Evaluation of patient reporting of adverse drug reactions to the UK 'Yellow Card Scheme': literature review, descriptive and qualitative analyses, and questionnaire surveys.Health Technol Assess. 2011 May;15(20):1-234, iii-iv. doi: 10.3310/hta15200. Health Technol Assess. 2011. PMID: 21545758 Review.
-
Inhaled steroids with and without regular formoterol for asthma: serious adverse events.Cochrane Database Syst Rev. 2019 Sep 25;9(9):CD006924. doi: 10.1002/14651858.CD006924.pub4. Cochrane Database Syst Rev. 2019. PMID: 31553802 Free PMC article.
Cited by
-
Sexual Dysfunction Induced by Antidepressants-A Pharmacovigilance Study Using Data from VigiBaseTM.Pharmaceuticals (Basel). 2024 Jun 24;17(7):826. doi: 10.3390/ph17070826. Pharmaceuticals (Basel). 2024. PMID: 39065677 Free PMC article.
-
Reporting Adverse Drug Events: A Comparison of an Online Patient Tool Versus Telephone-Based Monitoring in Community Pharmacy Patients in the Netherlands.Drug Saf. 2025 Jun 14. doi: 10.1007/s40264-025-01571-4. Online ahead of print. Drug Saf. 2025. PMID: 40517185
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

