Safety, Tolerability, Pharmacokinetics and Initial Pharmacodynamics of a Subcommissural Organ-Spondin-Derived Peptide: A Randomized, Placebo-Controlled, Double-Blind, Single Ascending Dose First-in-Human Study
- PMID: 35779189
- PMCID: PMC9338184
- DOI: 10.1007/s40120-022-00380-6
Safety, Tolerability, Pharmacokinetics and Initial Pharmacodynamics of a Subcommissural Organ-Spondin-Derived Peptide: A Randomized, Placebo-Controlled, Double-Blind, Single Ascending Dose First-in-Human Study
Abstract
Introduction: This randomized, double-blind, placebo-controlled study in healthy volunteers assessed the safety, tolerability, and pharmacokinetics of single ascending doses of intravenously administered NX210-a linear peptide derived from subcommissural organ-spondin-and explored the effects on blood/urine biomarkers and cerebral activity.
Methods: Participants in five cohorts (n = 8 each) were randomized to receive a single intravenous dose of NX210 (n = 6 each) (0.4, 1.25, 2.5, 5, and 10 mg/kg) or placebo (n = 2 each); in total, 10 and 29 participants received placebo and NX210, respectively. Blood samples were collected for pharmacokinetics within 180 min post dosing. Plasma and urine were collected from participants (cohorts: 2.5, 5, and 10 mg/kg) for biomarker analysis and electroencephalography (EEG) recordings within 48 h post dosing. Safety/tolerability and pharmacokinetic data were assessed before ascending to the next dose.
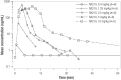
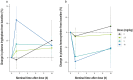
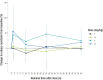
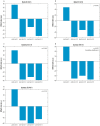
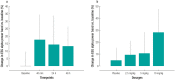
Results: The study included 39 participants. All dosages were safe and well tolerated. All treatment-emergent adverse events (n = 17) were of mild severity and resolved spontaneously (except one with unknown outcome). Twelve treatment-emergent adverse events (70.6%) were deemed drug related; seven of those (58.3%) concerned nervous system disorders (dizziness, headache, and somnolence). The pharmacokinetic analysis indicated a short half-life in plasma (6-20 min), high apparent volume of distribution (1870-4120 L), and rapid clearance (7440-16,400 L/h). In plasma, tryptophan and homocysteine showed dose-related increase and decrease, respectively. No drug dose effect was found for the glutamate or glutamine plasma biomarkers. Nevertheless, decreased blood glutamate and increased glutamine were observed in participants treated with NX210 versus placebo. EEG showed a statistically significant decrease in beta and gamma bands and a dose-dependent increasing trend in alpha bands. Pharmacodynamics effects were sustained for several hours (plasma) or 48 h (urine and EEG).
Conclusion: NX210 is safe and well tolerated and may exert beneficial effects on the central nervous system, particularly in terms of cognitive processing.
Keywords: Alzheimer’s disease; Cognitive processing; Intravenous administration; NX210 peptide; Subcommissural organ-spondin.
© 2022. The Author(s).
Figures
Similar articles
-
Safety, Tolerability and Pharmacokinetic-Pharmacodynamic Relationship of NX210c Peptide in Healthy Elderly Volunteers: Randomized, Placebo-Controlled, Double-Blind, Multiple Ascending Dose Study.Neurol Ther. 2025 Feb;14(1):357-377. doi: 10.1007/s40120-024-00691-w. Epub 2024 Dec 21. Neurol Ther. 2025. PMID: 39708220 Free PMC article.
-
Safety, Tolerability, Pharmacokinetics and Quantitative Electroencephalography Assessment of ACD856, a Novel Positive Allosteric Modulator of Trk-Receptors Following Multiple Doses in Healthy Subjects.J Prev Alzheimers Dis. 2023;10(4):778-789. doi: 10.14283/jpad.2023.89. J Prev Alzheimers Dis. 2023. PMID: 37874100 Clinical Trial.
-
Mitoxantrone: a review of its use in multiple sclerosis.CNS Drugs. 2004;18(6):379-96. doi: 10.2165/00023210-200418060-00010. CNS Drugs. 2004. PMID: 15089110 Review.
-
A Phase I, Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose, Multiple Dose, and Food Effect Trial of the Safety, Tolerability and Pharmacokinetics of Highly Purified Cannabidiol in Healthy Subjects.CNS Drugs. 2018 Nov;32(11):1053-1067. doi: 10.1007/s40263-018-0578-5. CNS Drugs. 2018. PMID: 30374683 Free PMC article. Clinical Trial.
-
Clevidipine: a review of its use in the management of acute hypertension.Am J Cardiovasc Drugs. 2009;9(2):117-34. doi: 10.2165/00129784-200909020-00006. Am J Cardiovasc Drugs. 2009. PMID: 19331440 Review.
Cited by
-
NX210c Peptide Promotes Glutamatergic Receptor-Mediated Synaptic Transmission and Signaling in the Mouse Central Nervous System.Int J Mol Sci. 2022 Aug 9;23(16):8867. doi: 10.3390/ijms23168867. Int J Mol Sci. 2022. PMID: 36012124 Free PMC article.
-
Safety, Tolerability and Pharmacokinetic-Pharmacodynamic Relationship of NX210c Peptide in Healthy Elderly Volunteers: Randomized, Placebo-Controlled, Double-Blind, Multiple Ascending Dose Study.Neurol Ther. 2025 Feb;14(1):357-377. doi: 10.1007/s40120-024-00691-w. Epub 2024 Dec 21. Neurol Ther. 2025. PMID: 39708220 Free PMC article.
-
NX210c drug candidate peptide strengthens mouse and human blood-brain barriers.Fluids Barriers CNS. 2024 Sep 27;21(1):76. doi: 10.1186/s12987-024-00577-x. Fluids Barriers CNS. 2024. PMID: 39334382 Free PMC article.
References
-
- Patterson C. World Alzheimer Report 2018. London: Alzheimer’s Disease International 2018. Published 21 September 2021. https://www.alzint.org/u/WorldAlzheimerReport2018.pdf. Accessed 29 May 2022.
-
- Boutajangout A, Frontera J, Debure L, Vedvyas A, Faustin A, Wisniewski T. Plasma biomarkers of neurodegeneration and neuroinflammation in hospitalized COVID-19 patients with and without new neurological symptoms. Alzheimer's Dement. 2021;17(Suppl. 5):e057892. doi: 10.1002/alz.057892. - DOI
-
- Sachdev PS, Lo JW, Crawford JD, et al. STROKOG (stroke and cognition consortium): an international consortium to examine the epidemiology, diagnosis, and treatment of neurocognitive disorders in relation to cerebrovascular disease. Alzheimers Dement (Amst) 2016;7:11–23. doi: 10.1016/j.dadm.2016.10.006. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

