An exploratory analysis of the response to ChAdOx1 nCoV-19 (AZD1222) vaccine in males and females
- PMID: 35779491
- PMCID: PMC9242842
- DOI: 10.1016/j.ebiom.2022.104128
An exploratory analysis of the response to ChAdOx1 nCoV-19 (AZD1222) vaccine in males and females
Abstract
Background: There are known differences in vaccine reactogenicity and immunogenicity by sex. Females have been shown to report greater reactogenicity and generate higher humoral and cellular immune responses than males following vaccination with several different vaccines. Whether this is also the case for COVID-19 vaccines is currently unknown, as COVID-19 vaccine study data disaggregated by sex are not routinely reported. Therefore, we have assessed the influence of sex on reactogenicity, immunogenicity and efficacy of COVID-19 vaccine ChAdOx1 nCoV-19.
Methods: Vaccine efficacy was assessed in 15169 volunteers enrolled into single-blind randomised controlled trials of ChAdOx1 nCoV-19 in Brazil and the UK, with the primary endpoint defined as nucleic acid amplification test (NAAT)-positive symptomatic SARS-CoV-2 infection. All participants were electronically randomised to receive two standard doses of vaccine or the control product. Logistic regression models were fitted to explore the effect of age and sex on reactogenicity, and linear models fitted to log-transformed values for immunogenicity data. Reactogenicity data were taken from self-reported diaries of 788 trial participants. Pseudovirus neutralisation assay data were available from 748 participants and anti-SARS-CoV-2 spike IgG assay data from 1543 participants.
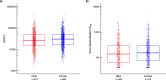
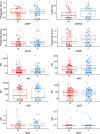
Findings: 7619 participants received ChAdOx1 nCoV-19 and 7550 received the control. Vaccine efficacy in participants after two doses of ChAdOx1 nCoV-19 (4243 females and 3376 males) was 66.1% (95% CI 55.9-73.9%) in males and 59.9% (95% CI 49.8-67.9%) in females; with no evidence of a difference in efficacy between the sexes (vaccine by sex interaction term P=0.3359). A small, statistically significant difference in anti-spike IgG was observed (adjusted GMR 1.14; 95% CI 1.04-1.26), with higher titres in females than males, but there were no statistically significant differences in other immunological endpoints. Whilst the majority of individuals reported at least one systemic reaction following a first dose of ChAdOx1 nCoV-19, females were twice as likely as males to report any systemic reaction after a first dose (OR 1.95; 95% CI 1.37-2.77). Measured fever of 38°C or above was reported in 5% of females and 1% of males following first doses. Headache and fatigue were the most commonly reported reactions in both sexes.
Interpretation: Our results show that there is no evidence of difference in efficacy of the COVID-19 vaccine ChAdOx1 nCoV-19 in males and females. Greater reactogenicity in females was not associated with any difference in vaccine efficacy.
Funding: Studies were registered with ISRCTN 90906759 (COV002) and ISRCTN 89951424 (COV003) and follow-up is ongoing. Funding was received from the UK Research and Innovation, Engineering and Physical Sciences Research Council, National Institute for Health Research, Coalition for Epidemic Preparedness Innovations, National Institute for Health Research Oxford Biomedical Research Centre, Chinese Academy of Medical Sciences Innovation Fund for Medical Science, Thames Valley and South Midlands NIHR Clinical Research Network, the Lemann Foundation, Rede D'Or, the Brava and Telles Foundation, the Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior, Brazil, and AstraZeneca.
Keywords: COVID-19; Clinical trials; Sex-differences; Vaccination.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests Oxford University has entered into a partnership with AstraZeneca for further development of ChAdOx1 nCoV-19. AstraZeneca reviewed the final manuscript before submission, but the authors retained editorial control. SCG is a cofounder of and shareholder in Vaccitech (collaborators in the early development of this vaccine candidate) and named as an inventor on a patent covering use of ChAdOx1-vectored vaccines (PCT/GB2012/000467) and a patent application covering this SARS-CoV-2 vaccine. TL is named as an inventor on a patent covering use of ChAdOx1-vectored vaccines (PCT/GB2012/000467) and was a consultant to Vaccitech. PMF is a consultant to Vaccitech. AJP is Chair of the UK Department of Health and Social Care's JCVI, but does not participate in policy advice on coronavirus vaccines, and was a member of the WHO Strategic Advisory Group of Experts (SAGE) until 2022. MNR has acted as PI on commercial vaccine trials sponsored by Astra Zeneca and VBI vaccines but not personally received payment for this work. MV has received grants from the NIHR and Bill and Melinda Gates Foundation not related to this work and participated on Data Safety Monitoring Boards for NIHR funded projects not related to this paper.
Figures



References
-
- Leong HN, Chan KP, Oon LLE, Koay ESC, Ng LC, Lee MA, et al. Clinical and laboratory findings of SARS in Singapore. Ann Acad Med Singapore. 2006;35(5):332–339. - PubMed
-
- Global Health 50/50, ‘Gender Equality: Flying blind in a time of crisis, The Global Health 50/50 Report 2021’. 151 pages. London, UK, 2021. Accessed online 22 June 2022.https://globalhealth5050.org/wp-content/uploads/Global-Health-5050-2021-....
-
- Heidari S and Goodman T. Critical sex and gender considerations for equitable research, development and delivery of covid-19 vaccines working paper. World Health Organisation, SAGE COVID-19 Working Group. 19th April 2021. 28 pages. Accessed 22 June 2022.https://cdn.who.int/media/docs/default-source/immunization/sage/covid/ge....
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

