Recapitulating infection, thermal sensitivity and antiviral treatment of seasonal coronaviruses in human airway organoids
- PMID: 35779493
- PMCID: PMC9240613
- DOI: 10.1016/j.ebiom.2022.104132
Recapitulating infection, thermal sensitivity and antiviral treatment of seasonal coronaviruses in human airway organoids
Abstract
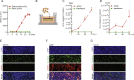
Background: Human seasonal coronaviruses usually cause mild upper-respiratory tract infection, but severe complications can occur in specific populations. Research into seasonal coronaviruses is limited and robust experimental models are largely lacking. This study aims to establish human airway organoids (hAOs)-based systems for seasonal coronavirus infection and to demonstrate their applications in studying virus-host interactions and therapeutic development.
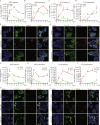
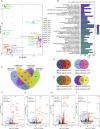
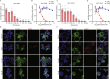
Methods: The infections of seasonal coronaviruses 229E, OC43 and NL63 in 3D cultured hAOs with undifferentiated or differentiated phenotypes were tested. The kinetics of virus replication and production was profiled at 33 °C and 37 °C. Genome-wide transcriptome analysis by RNA sequencing was performed in hAOs under various conditions. The antiviral activity of molnupiravir and remdesivir, two approved medications for treating COVID19, was tested.
Findings: HAOs efficiently support the replication and infectious virus production of seasonal coronaviruses 229E, OC43 and NL63. Interestingly, seasonal coronaviruses replicate much more efficiently at 33 °C compared to 37 °C, resulting in over 10-fold higher levels of viral replication. Genome-wide transcriptomic analyses revealed distinct patterns of infection-triggered host responses at 33 °C compared to 37 °C temperature. Treatment of molnupiravir and remdesivir dose-dependently inhibited the replication of 229E, OC43 and NL63 in hAOs.
Interpretation: HAOs are capable of modeling 229E, OC43 and NL63 infections. The intriguing finding that lower temperature resembling that in the upper respiratory tract favors viral replication may help to better understand the pathogenesis and transmissibility of seasonal coronaviruses. HAOs-based innovative models shall facilitate the research and therapeutic development against seasonal coronavirus infections.
Funding: This research is supported by funding of a VIDI grant (No. 91719300) from the Netherlands Organization for Scientific Research and the Dutch Cancer Society Young Investigator Grant (10140) to Q.P., and the ZonMw COVID project (114025011) from the Netherlands Organization for Health Research and Development to R.R.
Keywords: Airway organoids; Antiviral therapy; Seasonal coronaviruses; Thermal sensitivity; Virus-host interactions.
Copyright © 2022 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests No conflict of interest.
Figures



Similar articles
-
Seasonal human coronaviruses OC43, 229E, and NL63 induce cell surface modulation of entry receptors and display host cell-specific viral replication kinetics.Microbiol Spectr. 2024 Jul 2;12(7):e0422023. doi: 10.1128/spectrum.04220-23. Epub 2024 Jun 12. Microbiol Spectr. 2024. PMID: 38864599 Free PMC article.
-
Immunosuppressants exert differential effects on pan-coronavirus infection and distinct combinatory antiviral activity with molnupiravir and nirmatrelvir.United European Gastroenterol J. 2023 Jun;11(5):431-447. doi: 10.1002/ueg2.12417. Epub 2023 May 25. United European Gastroenterol J. 2023. PMID: 37226653 Free PMC article.
-
Viral polymerase binding and broad-spectrum antiviral activity of molnupiravir against human seasonal coronaviruses.Virology. 2021 Dec;564:33-38. doi: 10.1016/j.virol.2021.09.009. Epub 2021 Oct 2. Virology. 2021. PMID: 34619630 Free PMC article.
-
Complicating Infections Associated with Common Endemic Human Respiratory Coronaviruses.Health Secur. 2021 Mar-Apr;19(2):195-208. doi: 10.1089/hs.2020.0067. Epub 2020 Nov 11. Health Secur. 2021. PMID: 33186086 Review.
-
An overview on the seven pathogenic human coronaviruses.Rev Med Virol. 2022 Mar;32(2):e2282. doi: 10.1002/rmv.2282. Epub 2021 Aug 2. Rev Med Virol. 2022. PMID: 34339073 Review.
Cited by
-
Systematic analysis and prediction of the burden of lower respiratory tract infections attribute to non-optimal temperature, 1990-2019.Front Public Health. 2024 Oct 18;12:1424657. doi: 10.3389/fpubh.2024.1424657. eCollection 2024. Front Public Health. 2024. PMID: 39494067 Free PMC article.
-
Combating pan-coronavirus infection by indomethacin through simultaneously inhibiting viral replication and inflammatory response.iScience. 2023 Aug 16;26(9):107631. doi: 10.1016/j.isci.2023.107631. eCollection 2023 Sep 15. iScience. 2023. PMID: 37664584 Free PMC article.
-
A nano-luciferase expressing human coronavirus OC43 for countermeasure development.Virus Res. 2024 Jan 2;339:199286. doi: 10.1016/j.virusres.2023.199286. Epub 2023 Dec 5. Virus Res. 2024. PMID: 38016504 Free PMC article.
-
Remdesivir for the Treatment of Human Coronavirus OC43 Encephalitis.J Med Virol. 2025 Mar;97(3):e70314. doi: 10.1002/jmv.70314. J Med Virol. 2025. PMID: 40108967 Free PMC article.
-
Macrophage-augmented organoids recapitulate the complex pathophysiology of viral diseases and enable development of multitarget therapeutics.Nat Biomed Eng. 2025 Jun 13. doi: 10.1038/s41551-025-01417-5. Online ahead of print. Nat Biomed Eng. 2025. PMID: 40514432
References
-
- Veiga A, Martins LG, Riediger I, Mazetto A, Debur MDC, Gregianini TS. More than just a common cold: endemic coronaviruses OC43, HKU1, NL63, and 229E associated with severe acute respiratory infection and fatality cases among healthy adults. J Med Virol. 2021;93(2):1002–1007. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

