Enoxaparin for primary thromboprophylaxis in symptomatic outpatients with COVID-19 (OVID): a randomised, open-label, parallel-group, multicentre, phase 3 trial
- PMID: 35779558
- PMCID: PMC9243568
- DOI: 10.1016/S2352-3026(22)00175-2
Enoxaparin for primary thromboprophylaxis in symptomatic outpatients with COVID-19 (OVID): a randomised, open-label, parallel-group, multicentre, phase 3 trial
Abstract
Background: COVID-19 is a viral prothrombotic respiratory infection. Heparins exert antithrombotic and anti-inflammatory effects, and might have antiviral properties. We aimed to investigate whether thromboprophylaxis with enoxaparin would prevent untoward hospitalisation and death in symptomatic, but clinically stable outpatients with COVID-19.
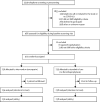
Methods: OVID was a randomised, open-label, parallel-group, investigator-initiated, phase 3 trial and was done at eight centres in Switzerland and Germany. Outpatients aged 50 years or older with acute COVID-19 were eligible if they presented with respiratory symptoms or body temperature higher than 37·5°C. Eligible participants underwent block-stratified randomisation (by age group 50-70 vs >70 years and by study centre) in a 1:1 ratio to receive either subcutaneous enoxaparin 40 mg once daily for 14 days versus standard of care (no thromboprophylaxis). The primary outcome was a composite of any untoward hospitalisation and all-cause death within 30 days of randomisation. Analysis of the efficacy outcomes was done in the intention-to-treat population. The primary safety outcome was major bleeding. The study was registered in ClinicalTrials.gov (NCT04400799) and has been completed.
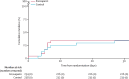
Findings: At the predefined formal interim analysis for efficacy (50% of total study population), the independent Data Safety Monitoring Board recommended early termination of the trial on the basis of predefined statistical criteria having considered the very low probability of showing superiority of thromboprophylaxis with enoxaparin for the primary outcome under the initial study design assumptions. Between Aug 15, 2020, and Jan 14, 2022, from 3319 participants prescreened, 472 were included in the intention-to-treat population and randomly assigned to receive enoxaparin (n=234) or standard of care (n=238). The median age was 57 years (IQR 53-62) and 217 (46%) were women. The 30-day risk of the primary outcome was similar in participants allocated to receive enoxaparin and in controls (8 [3%] of 234 vs 8 [3%] of 238; adjusted relative risk 0·98; 95% CI 0·37-2·56; p=0·96). All hospitalisations were related to COVID-19. No deaths were reported during the study. No major bleeding events were recorded. Eight serious adverse events were recorded in the enoxaparin group versus nine in the control group.
Interpretation: These findings suggest thromboprophylaxis with enoxaparin does not reduce early hospitalisations and deaths among outpatients with symptomatic COVID-19. Futility of the treatment under the initial study design assumptions could not be conclusively assessed owing to under-representation of older patients and consequent low event rates.
Funding: SNSF (National Research Programme COVID-19 NRP78: 198352), University Hospital Zurich, University of Zurich, Dr-Ing Georg Pollert (Berlin), Johanna Dürmüller-Bol Foundation.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests SB reports institutional research grants from Concept Medical, Bard, Bentley, Boston Scientific, INARI, Sanofi, and Bayer; and personal fees from Concept Medical, Bayer, Boston Scientific, and INARI. DV, UH, AG, NRS, GC, FM, MR, and TS do not report any conflicts of interest. BG reports non-financial support and funding for an accredited continuing medical education programme from Axonlab, and Thermo Fisher Scientific; personal fees and funding for an accredited continuing medical education programme from Alnylam, Pfizer, and Sanofi; funding for an accredited continuing medical education programme from Bayer, Bristol Myers Squibb, Daiichi-Sankyo, Takeda, Octapharma, SOBI, Janssen, Novo Nordisk, Mitsubishi Pfizer, Tanabe Pharma, outside the submitted work. SVK reports grants or contracts from Bayer AG; consulting fees from Bayer, Daiichi Sankyo, and Boston Scientific; and payment or honoraria from Bayer, INARI Medical, MSD, Pfizer, and Bristol-Myers Squibb. SS reports research grants from Edwards Lifesciences to the institution, research grants from Medtronic to the institution, research grants from Boston Scientific to the institution, research grants from Abbott to the institution, personal fees from Boston Scientific, from Teleflex, from BTG –Boston Scientific outside the submitted work. HRE reports speaker honoraria from Daichi-Sankyo, and Bayer. DS reports employment by Sanofi-Aventis Switzerland. DD reports research support from German Research Foundation, CytoSorbents, Haemonetic; consulting and speaker's fees from Bayer Healthcare, Daiichi Sankyo, LEO Pharma, AstraZeneca, Boston Scientific, and BMS–Pfizer. NK reports institutional research grants from Concept Medical, Bard, Bentley, Boston Scientific, INARI, Sanofi, and Bayer; and personal fees from Concept Medical, Bayer, Boston Scientific, and INARI.
Figures
Comment in
-
Antithrombotic prophylaxis for symptomatic outpatients with COVID-19: less is consistently more.Lancet Haematol. 2022 Aug;9(8):e551-e553. doi: 10.1016/S2352-3026(22)00205-8. Epub 2022 Jun 30. Lancet Haematol. 2022. PMID: 35779559 Free PMC article. No abstract available.
References
-
- Young E. The anti-inflammatory effects of heparin and related compounds. Thromb Res. 2008;122:743–752. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical