Spermidine reduces neuroinflammation and soluble amyloid beta in an Alzheimer's disease mouse model
- PMID: 35780157
- PMCID: PMC9250727
- DOI: 10.1186/s12974-022-02534-7
Spermidine reduces neuroinflammation and soluble amyloid beta in an Alzheimer's disease mouse model
Abstract
Background: Deposition of amyloid beta (Aβ) and hyperphosphorylated tau along with glial cell-mediated neuroinflammation are prominent pathogenic hallmarks of Alzheimer's disease (AD). In recent years, impairment of autophagy has been identified as another important feature contributing to AD progression. Therefore, the potential of the autophagy activator spermidine, a small body-endogenous polyamine often used as dietary supplement, was assessed on Aβ pathology and glial cell-mediated neuroinflammation.
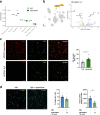
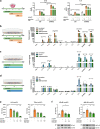
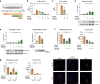
Results: Oral treatment of the amyloid prone AD-like APPPS1 mice with spermidine reduced neurotoxic soluble Aβ and decreased AD-associated neuroinflammation. Mechanistically, single nuclei sequencing revealed AD-associated microglia to be the main target of spermidine. This microglia population was characterized by increased AXL levels and expression of genes implicated in cell migration and phagocytosis. A subsequent proteome analysis of isolated microglia confirmed the anti-inflammatory and cytoskeletal effects of spermidine in APPPS1 mice. In primary microglia and astrocytes, spermidine-induced autophagy subsequently affected TLR3- and TLR4-mediated inflammatory processes, phagocytosis of Aβ and motility. Interestingly, spermidine regulated the neuroinflammatory response of microglia beyond transcriptional control by interfering with the assembly of the inflammasome.
Conclusions: Our data highlight that the autophagy activator spermidine holds the potential to enhance Aβ degradation and to counteract glia-mediated neuroinflammation in AD pathology.
Keywords: Alzheimer’s disease; Astrocytes; Autophagy; Dietary supplement; Liquid chromatography tandem mass spectrometry; Microglia; Neuroinflammation; Phagocytosis; Single nuclei sequencing; Spermidine.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Montoliu-Gaya L, Mulder SD, Herrebout MAC, Baayen JC, Villegas S, Veerhuis R. Aβ-oligomer uptake and the resulting inflammatory response in adult human astrocytes are precluded by an anti-Aβ single chain variable fragment in combination with an apoE mimetic peptide. Mol Cell Neurosci. 2018;89:49–59. doi: 10.1016/j.mcn.2018.03.015. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

