Quantitative Interstitial Abnormality Progression and Outcomes in the Genetic Epidemiology of COPD and Pittsburgh Lung Screening Study Cohorts
- PMID: 35780812
- PMCID: PMC9859724
- DOI: 10.1016/j.chest.2022.06.030
Quantitative Interstitial Abnormality Progression and Outcomes in the Genetic Epidemiology of COPD and Pittsburgh Lung Screening Study Cohorts
Abstract
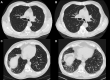
Background: The risk factors and clinical outcomes of quantitative interstitial abnormality progression over time have not been characterized.
Research questions: What are the associations of quantitative interstitial abnormality progression with lung function, exercise capacity, and mortality? What are the demographic and genetic risk factors for quantitative interstitial abnormality progression?
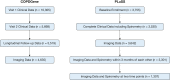
Study design and methods: Quantitative interstitial abnormality progression between visits 1 and 2 was assessed from 4,635 participants in the Genetic Epidemiology of COPD (COPDGene) cohort and 1,307 participants in the Pittsburgh Lung Screening Study (PLuSS) cohort. We used multivariable linear regression to determine the risk factors for progression and the longitudinal associations between progression and FVC and 6-min walk distance, and Cox regression models for the association with mortality.
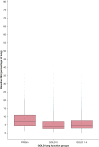
Results: Age at enrollment, female sex, current smoking status, and the MUC5B minor allele were associated with quantitative interstitial abnormality progression. Each percent annual increase in quantitative interstitial abnormalities was associated with annual declines in FVC (COPDGene: 8.5 mL/y; 95% CI, 4.7-12.4 mL/y; P < .001; PLuSS: 9.5 mL/y; 95% CI, 3.7-15.4 mL/y; P = .001) and 6-min walk distance, and increased mortality (COPDGene: hazard ratio, 1.69; 95% CI, 1.34-2.12; P < .001; PLuSS: hazard ratio, 1.28; 95% CI, 1.10-1.49; P = .001).
Interpretation: The objective, longitudinal measurement of quantitative interstitial abnormalities may help identify people at greatest risk for adverse events and most likely to benefit from early intervention.
Keywords: 6-min walk test; interstitial lung disease; pulmonary fibrosis; pulmonary function test; radiology.
Copyright © 2022 American College of Chest Physicians. Published by Elsevier Inc. All rights reserved.
Figures
References
Publication types
MeSH terms
Grants and funding
- R01 AR077607/AR/NIAMS NIH HHS/United States
- R01 HL149877/HL/NHLBI NIH HHS/United States
- T32 HL007633/HL/NHLBI NIH HHS/United States
- R01 HL155522/HL/NHLBI NIH HHS/United States
- U01 HL089897/HL/NHLBI NIH HHS/United States
- R01 HL116931/HL/NHLBI NIH HHS/United States
- R21 HL140422/HL/NHLBI NIH HHS/United States
- R01 HL135142/HL/NHLBI NIH HHS/United States
- F32 HL162318/HL/NHLBI NIH HHS/United States
- R01 HL137927/HL/NHLBI NIH HHS/United States
- K08 HL145118/HL/NHLBI NIH HHS/United States
- T32 HL007427/HL/NHLBI NIH HHS/United States
- K23 HL136905/HL/NHLBI NIH HHS/United States
- R01 HL133137/HL/NHLBI NIH HHS/United States
- P50 CA090440/CA/NCI NIH HHS/United States
- R03 HL148484/HL/NHLBI NIH HHS/United States
- K23 HL141651/HL/NHLBI NIH HHS/United States
- K23 HL119558/HL/NHLBI NIH HHS/United States
- R01 HL147148/HL/NHLBI NIH HHS/United States
- U01 HL089856/HL/NHLBI NIH HHS/United States
- R21 LM013670/LM/NLM NIH HHS/United States
- R01 HL111024/HL/NHLBI NIH HHS/United States
- R01 HL122477/HL/NHLBI NIH HHS/United States
- R01 HL149861/HL/NHLBI NIH HHS/United States
- K24 HL138188/HL/NHLBI NIH HHS/United States
- P01 HL114501/HL/NHLBI NIH HHS/United States

