Imaging of Central Nervous System Involvement in Pediatric Hematologic Disorders
- PMID: 35781229
- PMCID: PMC9131816
- DOI: 10.5152/TurkArchPediatr.2022.22085
Imaging of Central Nervous System Involvement in Pediatric Hematologic Disorders
Abstract
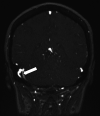
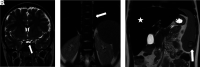
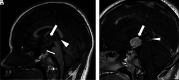
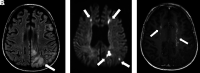
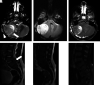
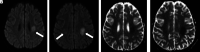
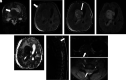
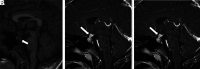
Hematologic disorders and malignancies are commonly encountered in children. The central nervous system is affected by many benign and malignant hematological diseases. Neurologic findings may occur due to central involvement of hematological disease or neurotoxic effects of treatment during the disease. At all these stages, radiological imaging and evaluation have a critical role. In this review article, we aim to describe the imaging findings of central nervous system involvement in pediatric hematologic diseases. This review was prepared based on the latest literature available in the PubMed database in the English language from inception to March 2022. The radiological images of the patients in our archive were obtained from the PACS (Picture Archiving and Communication Systems) to set an example for the diseases described in the text.
Figures












Similar articles
-
Electronic teaching files: seven-year experience using a commercial picture archiving and communication system.J Digit Imaging. 2001 Jun;14(2 Suppl 1):125-7. doi: 10.1007/BF03190314. J Digit Imaging. 2001. PMID: 11442071 Free PMC article.
-
Imaging of Nervous System Involvement in Hematologic Malignancies: What Radiologists Need to Know.AJR Am J Roentgenol. 2015 Sep;205(3):604-17. doi: 10.2214/AJR.14.14092. AJR Am J Roentgenol. 2015. PMID: 26295649 Review.
-
An integrated picture archiving and communications system-radiology information system in a radiological department.J Digit Imaging. 1993 Feb;6(1):16-24. doi: 10.1007/BF03168413. J Digit Imaging. 1993. PMID: 8439578
-
Picture archiving and communication systems (PACS) for radiological images: state of the art.Crit Rev Diagn Imaging. 1988;28(4):383-427. Crit Rev Diagn Imaging. 1988. PMID: 3053047 Review.
-
Hospital, radiology, and picture archiving and communication systems.Vet Radiol Ultrasound. 2008 Jan-Feb;49(1 Suppl 1):S19-28. doi: 10.1111/j.1740-8261.2007.00329.x. Vet Radiol Ultrasound. 2008. PMID: 18283982
References
-
- McDonald J, Stevenson DA. Hereditary hemorrhagic telangiectasia. GeneReviews® [internet]; 2021. https://www.ncbi.nlm.nih.gov/books/NBK1351/. Accessed 2022 Apr 8.
LinkOut - more resources
Full Text Sources
