Novel intravesical bacterial immunotherapy induces rejection of BCG-unresponsive established bladder tumors
- PMID: 35781395
- PMCID: PMC9252205
- DOI: 10.1136/jitc-2021-004325
Novel intravesical bacterial immunotherapy induces rejection of BCG-unresponsive established bladder tumors
Abstract
Background: Intravesical BCG is the gold-standard therapy for non-muscle invasive bladder cancer (NMIBC); however, it still fails in a significant proportion of patients, so improved treatment options are urgently needed.
Methods: Here, we compared BCG antitumoral efficacy with another live attenuated mycobacteria, MTBVAC, in an orthotopic mouse model of bladder cancer (BC). We aimed to identify both bacterial and host immunological factors to understand the antitumoral mechanisms behind effective bacterial immunotherapy for BC.
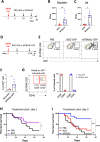
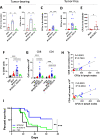
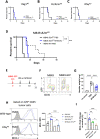
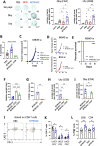
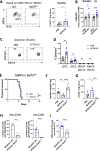
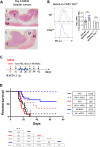
Results: We found that the expression of the BCG-absent proteins ESAT6/CFP10 by MTBVAC was determinant in mediating bladder colonization by the bacteria, which correlated with augmented antitumoral efficacy. We further analyzed the mechanism of action of bacterial immunotherapy and found that it critically relied on the adaptive cytotoxic response. MTBVAC enhanced both tumor antigen-specific CD4+ and CD8+ T-cell responses, in a process dependent on stimulation of type 1 conventional dendritic cells. Importantly, improved intravesical bacterial immunotherapy using MBTVAC induced eradication of fully established bladder tumors, both as a monotherapy and specially in combination with the immune checkpoint inhibitor antiprogrammed cell death ligand 1 (anti PD-L1).
Conclusion: These results contribute to the understanding of the mechanisms behind successful bacterial immunotherapy against BC and characterize a novel therapeutic approach for BCG-unresponsive NMIBC cases.
Keywords: Adaptive Immunity; Dendritic Cells; Immunogenicity, Vaccine; Immunotherapy; Urinary Bladder Neoplasms.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SU, EP, ER, CM and NA are coinventors of the patent 'Compositions for use as a prophylactic agent to those at risk of infection of tuberculosis, or as secondary agents for treating infected tuberculosis patients' held by the University of Zaragoza and Biofabri. CM is inventor of the patent 'Tuberculosis vaccine' held by the University of Zaragoza. There are no other conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
