Identification of ELK1 interacting peptide segments in the androgen receptor
- PMID: 35781489
- PMCID: PMC9422957
- DOI: 10.1042/BCJ20220297
Identification of ELK1 interacting peptide segments in the androgen receptor
Abstract
Prostate cancer (PCa) growth requires tethering of the androgen receptor (AR) to chromatin by the ETS domain transcription factor ELK1 to coactivate critical cell proliferation genes. Disruption of the ELK1-AR complex is a validated potential means of therapeutic intervention in PCa. AR associates with ELK1 by coopting its two ERK docking sites, through the amino-terminal domain (A/B domain) of AR. Using a mammalian two-hybrid assay, we have now functionally mapped amino acids within the peptide segments 358-457 and 514-557 in the A/B domain as required for association with ELK1. The mapping data were validated by GST (glutathione S-transferase)-pulldown and BRET (bioluminescence resonance energy transfer) assays. Comparison of the relative contributions of the interacting motifs/segments in ELK1 and AR to coactivation of ELK1 by AR suggested a parallel mode of binding of AR and ELK1 polypeptides. Growth of PCa cells was partially inhibited by deletion of the upstream segment in AR and nearly fully inhibited by deletion of the downstream segment. Our studies have identified two peptide segments in AR that mediate the functional association of AR with its two docking sites in ELK1. Identification of the ELK1 recognition sites in AR should enable further structural studies of the ELK1-AR interaction and rational design of small molecule drugs to disrupt this interaction.
Keywords: D-box; ELK1; ERK; FxFP motif; androgen receptor; prostate cancer.
© 2022 The Author(s). Published by Portland Press Limited on behalf of the Biochemical Society.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflicts of interest.
Figures


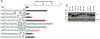
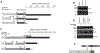


References
-
- Linja MJ, Savinainen KJ, Saramäki OR, Tammela TL, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61(9):3550–5. - PubMed
-
- Shafi AA, Yen AE, Weigel NL. Androgen receptors in hormone-dependent and castration-resistant prostate cancer. Pharmacol Ther. 2013;140(3):223–38. - PubMed
-
- Zegarra-Moro OL, Schmidt LJ, Huang H, Tindall DJ. Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res. 2002;62(4):1008–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

