Optical molecular imaging and theranostics in neurological diseases based on aggregation-induced emission luminogens
- PMID: 35781601
- PMCID: PMC9606072
- DOI: 10.1007/s00259-022-05894-7
Optical molecular imaging and theranostics in neurological diseases based on aggregation-induced emission luminogens
Abstract
Optical molecular imaging and image-guided theranostics benefit from special and specific imaging agents, for which aggregation-induced emission luminogens (AIEgens) have been regarded as good candidates in many biomedical applications. They display a large Stokes shift, high quantum yield, good biocompatibility, and resistance to photobleaching. Neurological diseases are becoming a substantial burden on individuals and society that affect over 50 million people worldwide. It is urgently needed to explore in more detail the brain structure and function, learn more about pathological processes of neurological diseases, and develop more efficient approaches for theranostics. Many AIEgens have been successfully designed, synthesized, and further applied for molecular imaging and image-guided theranostics in neurological diseases such as cerebrovascular disease, neurodegenerative disease, and brain tumor, which help us understand more about the pathophysiological state of brain through noninvasive optical imaging approaches. Herein, we focus on representative AIEgens investigated on brain vasculature imaging and theranostics in neurological diseases including cerebrovascular disease, neurodegenerative disease, and brain tumor. Considering different imaging modalities and various therapeutic functions, AIEgens have great potential to broaden neurological research and meet urgent needs in clinical practice. It will be inspiring to develop more practical and versatile AIEgens as molecular imaging agents for preclinical and clinical use on neurological diseases.
Keywords: Aggregation-induced emission; Brain vasculature; Fluorescence imaging; Neurological diseases; Theranostics.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

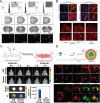
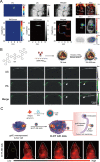
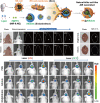
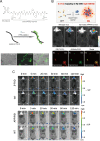
Similar articles
-
Hypoxia-responsive AIEgens for precise disease theranostics.Luminescence. 2024 Jan;39(1):e4659. doi: 10.1002/bio.4659. Luminescence. 2024. PMID: 38286609 Review.
-
The fast-growing field of photo-driven theranostics based on aggregation-induced emission.Chem Soc Rev. 2022 Mar 21;51(6):1983-2030. doi: 10.1039/d1cs01138c. Chem Soc Rev. 2022. PMID: 35226010 Review.
-
Aggregation-Induced Emission (AIE) Dots: Emerging Theranostic Nanolights.Acc Chem Res. 2018 Jun 19;51(6):1404-1414. doi: 10.1021/acs.accounts.8b00060. Epub 2018 May 7. Acc Chem Res. 2018. PMID: 29733571
-
AIEgens for biological process monitoring and disease theranostics.Biomaterials. 2017 Nov;146:115-135. doi: 10.1016/j.biomaterials.2017.09.004. Epub 2017 Sep 4. Biomaterials. 2017. PMID: 28915410 Review.
-
Intracellularly manipulable aggregation of the aggregation-induced emission luminogens.Biosens Bioelectron. 2025 Jan 1;267:116800. doi: 10.1016/j.bios.2024.116800. Epub 2024 Sep 19. Biosens Bioelectron. 2025. PMID: 39341072 Review.
Cited by
-
Advances in optical molecular imaging for neural visualization.Front Bioeng Biotechnol. 2023 Aug 21;11:1250594. doi: 10.3389/fbioe.2023.1250594. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37671191 Free PMC article. Review.
-
Current status and future prospects of molecular imaging in targeting the tumor immune microenvironment.Front Immunol. 2025 Jan 22;16:1518555. doi: 10.3389/fimmu.2025.1518555. eCollection 2025. Front Immunol. 2025. PMID: 39911388 Free PMC article. Review.
-
Quasi-dendritic sulfonate-based organic small molecule for high-quality NIR-II bone-targeted imaging.J Nanobiotechnology. 2023 Jul 19;21(1):230. doi: 10.1186/s12951-023-01999-9. J Nanobiotechnology. 2023. PMID: 37468990 Free PMC article.
-
Research hotspots and development trends in molecular imaging of glioma (2014-2024): A bibliometric review.Medicine (Baltimore). 2025 Jun 20;104(25):e42862. doi: 10.1097/MD.0000000000042862. Medicine (Baltimore). 2025. PMID: 40550035 Free PMC article. Review.
References
-
- James ML, Gambhir SS. A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev. 2012;92:897–965. - PubMed
-
- Böhmer VI, Szymanski W, Feringa BL, Elsinga PH. Multivalent probes in molecular imaging: reality or future? Trends Mol Med. 2021;27:379–393. - PubMed
-
- Strafella AP, Bohnen NI, Perlmutter JS, Eidelberg D, Pavese N, Van Eimeren T, et al. Molecular imaging to track Parkinson's disease and atypical parkinsonisms: new imaging frontiers. Mov Disord. 2017;32:181–192. - PubMed
-
- Zhou Z, Lu ZR. Molecular imaging of the tumor microenvironment. Adv Drug Deliv Rev. 2017;113:24–48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

