Population Pharmacokinetic Modelling of Intravenous Immunoglobulin Treatment in Patients with Guillain-Barré Syndrome
- PMID: 35781631
- PMCID: PMC9439991
- DOI: 10.1007/s40262-022-01136-z
Population Pharmacokinetic Modelling of Intravenous Immunoglobulin Treatment in Patients with Guillain-Barré Syndrome
Abstract
Background and objective: Intravenous immunoglobulin (IVIg) at a standard dosage is the treatment of choice for Guillain-Barré syndrome. The pharmacokinetics, however, is highly variable between patients, and a rapid clearance of IVIg is associated with poor recovery. We aimed to develop a model to predict the pharmacokinetics of a standard 5-day IVIg course (0.4 g/kg/day) in patients with Guillain-Barré syndrome.
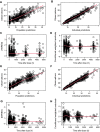
Methods: Non-linear mixed-effects modelling software (NONMEM®) was used to construct a pharmacokinetic model based on a model-building cohort of 177 patients with Guillain-Barré syndrome, with a total of 589 sequential serum samples tested for total immunoglobulin G (IgG) levels, and evaluated on an independent validation cohort that consisted of 177 patients with Guillain-Barré syndrome with 689 sequential serum samples.
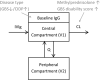
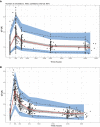
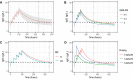
Results: The final two-compartment model accurately described the daily increment in serum IgG levels during a standard IVIg course; the initial rapid fall and then a gradual decline to steady-state levels thereafter. The covariates that increased IgG clearance were a more severe disease (as indicated by the Guillain-Barré syndrome disability score) and concomitant methylprednisolone treatment. When the current dosing regimen was simulated, the percentage of patients who reached a target ∆IgG > 7.3 g/L at 2 weeks decreased from 74% in mildly affected patients to only 33% in the most severely affected and mechanically ventilated patients (Guillain-Barré syndrome disability score of 5).
Conclusions: This is the first population-pharmacokinetic model for standard IVIg treatment in Guillain-Barré syndrome. The model provides a new tool to predict the pharmacokinetics of alternative regimens of IVIg in Guillain-Barré syndrome to design future trials and personalise treatment.
© 2022. The Author(s).
Conflict of interest statement
P.A.D. received honoraria and/or unrestricted research support from the Prinses Beatrix Spierfonds, Janivo Stichting, Baxalta, Grifols, Shire, Hans Biopharma, Kedrion, Octapharma and Sanquin Plasma Pharmaceuticals. B.C.J. received unrestricted research support from the Netherlands Organization for Health Research and Development, Erasmus MC, Prinses Beatrix Spierfonds, GBS-CIDP Foundation International, Baxalta, CSL-Behring, Grifols, Annexon and Hansa Biopharma. The other authors have no conflicts of interest that are directly relevant to the contents of this study.
Figures
Similar articles
-
IgG Fc N-glycosylation in Guillain-Barré syndrome treated with immunoglobulins.J Proteome Res. 2014 Mar 7;13(3):1722-30. doi: 10.1021/pr401213z. Epub 2014 Feb 17. J Proteome Res. 2014. PMID: 24533874 Clinical Trial.
-
Second intravenous immunoglobulin dose in patients with Guillain-Barré syndrome with poor prognosis (SID-GBS): a double-blind, randomised, placebo-controlled trial.Lancet Neurol. 2021 Apr;20(4):275-283. doi: 10.1016/S1474-4422(20)30494-4. Epub 2021 Mar 17. Lancet Neurol. 2021. PMID: 33743237 Clinical Trial.
-
Pharmacokinetics of intravenous immunoglobulin and outcome in Guillain-Barré syndrome.Ann Neurol. 2009 Nov;66(5):597-603. doi: 10.1002/ana.21737. Ann Neurol. 2009. PMID: 19938102 Clinical Trial.
-
Treatment of Guillain-Barré syndrome: a review.Inflamm Allergy Drug Targets. 2012 Aug 1;11(4):330-4. doi: 10.2174/187152812800959059. Inflamm Allergy Drug Targets. 2012. PMID: 22452606 Review.
-
Novel therapeutic approaches to Guillain-Barré syndrome.Expert Opin Investig Drugs. 2000 Oct;9(10):2307-18. doi: 10.1517/13543784.9.10.2307. Expert Opin Investig Drugs. 2000. PMID: 11060808 Review.
Cited by
-
Guillain-Barré syndrome.Nat Rev Dis Primers. 2024 Dec 19;10(1):97. doi: 10.1038/s41572-024-00580-4. Nat Rev Dis Primers. 2024. PMID: 39702645
-
Dose-exposure-efficacy response of intravenous immunoglobulin G 10% in multifocal motor neuropathy.Ann Clin Transl Neurol. 2024 Aug;11(8):1977-1987. doi: 10.1002/acn3.52098. Epub 2024 Jul 8. Ann Clin Transl Neurol. 2024. PMID: 38978354 Free PMC article. Clinical Trial.
-
If it does not help, it might hurt: Pharmacodynamics of a second IVIg course in Guillain-Barré syndrome.Ann Clin Transl Neurol. 2025 May;12(5):966-975. doi: 10.1002/acn3.52313. Epub 2025 Mar 14. Ann Clin Transl Neurol. 2025. PMID: 40084606 Free PMC article. Clinical Trial.
-
Genetic variation of low-to-medium-affinity Fc-gamma receptors in Guillain-Barré syndrome.J Neurol. 2025 Jul 1;272(7):487. doi: 10.1007/s00415-025-13216-8. J Neurol. 2025. PMID: 40593358 Free PMC article.
References
-
- van den Berg B, Walgaard C, Drenthen J, et al. Guillain–Barre syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. 2014;10(8):469–482. - PubMed
-
- Willison HJ, Jacobs BC, van Doorn PA. Guillain–Barre syndrome. Lancet. 2016;388(10045):717–727. - PubMed
-
- van der Meche FG, Schmitz PI. A randomized trial comparing intravenous immune globulin and plasma exchange in Guillain–Barre syndrome. Dutch Guillain–Barre Study Group. N Engl J Med. 1992;326(17):1123–1129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

