Heterogeneity in IgG-CD16 signaling in infectious disease outcomes
- PMID: 35781671
- PMCID: PMC9539944
- DOI: 10.1111/imr.13109
Heterogeneity in IgG-CD16 signaling in infectious disease outcomes
Abstract
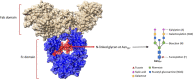
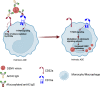
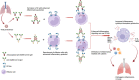
In this review, we discuss how IgG antibodies can modulate inflammatory signaling during viral infections with a focus on CD16a-mediated functions. We describe the structural heterogeneity of IgG antibody ligands, including subclass and glycosylation that impact binding by and downstream activity of CD16a, as well as the heterogeneity of CD16a itself, including allele and expression density. While inflammation is a mechanism required for immune homeostasis and resolution of acute infections, we focus here on two infectious diseases that are driven by pathogenic inflammatory responses during infection. Specifically, we review and discuss the evolving body of literature showing that afucosylated IgG immune complex signaling through CD16a contributes to the overwhelming inflammatory response that is central to the pathogenesis of severe forms of dengue disease and coronavirus disease 2019 (COVID-19).
Keywords: COVID-19; Fc afucosylation; IgG1 antibodies; dengue disease; effector functions.
© 2022 The Authors. Immunological Reviews published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

Similar articles
-
A single amino acid distorts the Fc γ receptor IIIb/CD16b structure upon binding immunoglobulin G1 and reduces affinity relative to CD16a.J Biol Chem. 2018 Dec 21;293(51):19899-19908. doi: 10.1074/jbc.RA118.005273. Epub 2018 Oct 25. J Biol Chem. 2018. PMID: 30361439 Free PMC article.
-
CD16a with oligomannose-type N-glycans is the only "low-affinity" Fc γ receptor that binds the IgG crystallizable fragment with high affinity in vitro.J Biol Chem. 2018 Oct 26;293(43):16842-16850. doi: 10.1074/jbc.RA118.004998. Epub 2018 Sep 13. J Biol Chem. 2018. PMID: 30213862 Free PMC article.
-
Antibody Fucosylation Lowers the FcγRIIIa/CD16a Affinity by Limiting the Conformations Sampled by the N162-Glycan.ACS Chem Biol. 2018 Aug 17;13(8):2179-2189. doi: 10.1021/acschembio.8b00342. Epub 2018 Jul 27. ACS Chem Biol. 2018. PMID: 30016589 Free PMC article.
-
Afucosylated IgG responses in humans - structural clues to the regulation of humoral immunity.Trends Immunol. 2022 Oct;43(10):800-814. doi: 10.1016/j.it.2022.08.001. Epub 2022 Aug 22. Trends Immunol. 2022. PMID: 36008258 Free PMC article. Review.
-
Terminal sugars of Fc glycans influence antibody effector functions of IgGs.Curr Opin Immunol. 2008 Aug;20(4):471-8. doi: 10.1016/j.coi.2008.06.007. Epub 2008 Jul 17. Curr Opin Immunol. 2008. PMID: 18606225 Review.
Cited by
-
Sialylated IgG induces the transcription factor REST in alveolar macrophages to protect against lung inflammation and severe influenza disease.Immunity. 2025 Jan 14;58(1):182-196.e10. doi: 10.1016/j.immuni.2024.10.002. Epub 2024 Nov 13. Immunity. 2025. PMID: 39541970
-
Association of Blood NK Cell Phenotype with the Severity of Liver Fibrosis in Patients with Chronic Viral Hepatitis C with Genotype 1 or 3.Diagnostics (Basel). 2024 Feb 21;14(5):472. doi: 10.3390/diagnostics14050472. Diagnostics (Basel). 2024. PMID: 38472945 Free PMC article.
-
Linking Effector Function to Antitumor Monoclonal Antibody Efficacy.J Immunol. 2024 Nov;213(10):1405-1406. doi: 10.4049/jimmunol.2400582. Epub 2024 Nov 15. J Immunol. 2024. PMID: 40008389 Free PMC article. No abstract available.
-
Understanding antibody-dependent enhancement in dengue: Are afucosylated IgG1s a concern?PLoS Pathog. 2023 Mar 30;19(3):e1011223. doi: 10.1371/journal.ppat.1011223. eCollection 2023 Mar. PLoS Pathog. 2023. PMID: 36996026 Free PMC article. No abstract available.
-
Immunity to SARS-CoV-2 infection.Immunol Rev. 2022 Aug;309(1):5-7. doi: 10.1111/imr.13117. Epub 2022 Jul 14. Immunol Rev. 2022. PMID: 35836369 Free PMC article. No abstract available.
References
-
- Knipe DM, Howley PM. Fields Virology. 6th ed. Wolters Kluwer/Lippincott Williams & Wilkins Health; 2013.
-
- Warren JS, Ward PA. Immune Complex Diseases. eLS; 2017:1‐9.
-
- Wang TT, Ravetch JV. Immune complexes: not just an innocent bystander in chronic viral infection. Immunity. 2015;42(2):213‐215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

