Analysis of therapy monitoring in the International Congenital Adrenal Hyperplasia Registry
- PMID: 35781728
- PMCID: PMC9796837
- DOI: 10.1111/cen.14796
Analysis of therapy monitoring in the International Congenital Adrenal Hyperplasia Registry
Abstract
Objective: Congenital adrenal hyperplasia (CAH) requires exogenous steroid replacement. Treatment is commonly monitored by measuring 17-OH progesterone (17OHP) and androstenedione (D4).
Design: Retrospective cohort study using real-world data to evaluate 17OHP and D4 in relation to hydrocortisone (HC) dose in CAH patients treated in 14 countries.
Patients: Pseudonymized data from children with 21-hydroxylase deficiency (21OHD) recorded in the International CAH Registry.
Measurements: Assessments between January 2000 and October 2020 in patients prescribed HC were reviewed to summarise biomarkers 17OHP and D4 and HC dose. Longitudinal assessment of measures was carried out using linear mixed-effects models (LMEM).
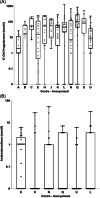
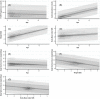
Results: Cohort of 345 patients, 52.2% female, median age 4.3 years (interquartile range: 3.1-9.2) were taking a median 11.3 mg/m2 /day (8.6-14.4) of HC. Median 17OHP was 35.7 nmol/l (3.0-104.0). Median D4 under 12 years was 0 nmol/L (0-2.0) and above 12 years was 10.5 nmol/L (3.9-21.0). There were significant differences in biomarker values between centres (p < 0.05). Correlation between D4 and 17OHP was good in multiple regression with age (p < 0.001, R2 = 0.29). In longitudinal assessment, 17OHP levels did not change with age, whereas D4 levels increased with age (p < 0.001, R2 = 0.08). Neither biomarker varied directly with dose or weight (p > 0.05). Multivariate LMEM showed HC dose decreasing by 1.0 mg/m2 /day for every 1 point increase in weight standard deviation score.
Discussion: Registry data show large variability in 17OHP and D4 between centres. 17OHP correlates with D4 well when accounting for age. Prescribed HC dose per body surface area decreased with weight gain.
Keywords: biomarkers; congenital adrenal hyperplasia; hydrocortisone; linear mixed-effects models.
© 2022 The Authors. Clinical Endocrinology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Sarafoglou K, Addo OY, Turcotte L, et al. Impact of hydrocortisone on adult height in congenital adrenal hyperplasia‐the Minnesota cohort. J Pediatr. 2014;164(5):1141‐1146.e1141. - PubMed
-
- Bacila I, Freeman N, Daniel E, et al. International practice of corticosteroid replacement therapy in congenital adrenal hyperplasia: data from the I‐CAH registry. Eur J Endocrinol. 2021;184(4):553‐563. - PubMed
-
- Clayton PE, Miller WL, Oberfield SE, Ritzen EM, Sippell WG, Speiser PW. Consensus statement on 21‐hydroxylase deficiency from the Lawson Wilkins pediatric endocrine society and the European society for paediatric endocrinology. J Clin Endocrinol Metab. 2002;87(9):4048‐4053. - PubMed

