The effect of mesenchymal stem cells improves the healing of burn wounds: a phase 1 dose-escalation clinical trial
- PMID: 35781931
- PMCID: PMC9247372
- DOI: 10.1177/20595131211070783
The effect of mesenchymal stem cells improves the healing of burn wounds: a phase 1 dose-escalation clinical trial
Erratum in
-
Erratum to The effect of mesenchymal stem cells improves the healing of burn wounds: A phase 1 dose-escalation clinical trial.Scars Burn Heal. 2022 Aug 11;8:20595131221118066. doi: 10.1177/20595131221118066. eCollection 2022 Jan-Dec. Scars Burn Heal. 2022. PMID: 35990795 Free PMC article.
Abstract
Background: Stem cell therapy holds promise to improve healing and stimulate tissue regeneration after burn injury. Preclinical evidence has supported this; however, clinical studies are lacking. We examined the application of bone marrow-derived mesenchymal stem cells (BM-MSC) to deep second-degree burn injuries using a two-dose escalation protocol.
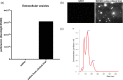
Methods: Ten individuals aged 18 years or older with deep second-degree burn wounds were enrolled. The first five patients were administered 2.5 × 10³ BM-MSC/cm2 to their wounds. After safety of the initial dose level was assessed, a second group of five patients was treated with a higher concentration of 5 × 10³ allogeneic BM-MSC/cm2. Safety was assessed clinically and by evaluating cytokine levels in mixed recipient lymphocyte/donor BM-MSC reactions (INFγ, IL-10 and TNFα). At each visit, we performed wound measurements and assessed wounds using a Patient and Observer Scar Assessment Scale (POSAS).
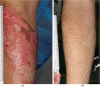
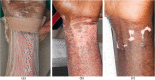
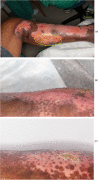
Results: All patients responded well to treatment, with 100% closure of wounds and minimal clinical evidence of fibrosis. No adverse reactions or evidence of rejection were observed for both dose levels. Patients receiving the first dose concentration had a wound closure rate of 3.64 cm2/day. Patients receiving the second dose concentration demonstrated a wound closure rate of 10.47 cm2/day. The difference in healing rates between the two groups was not found to be statistically significant (P = 0.17).
Conclusion: BM-MSC appear beneficial in optimising wound healing in patients with deep second-degree burn wounds. Adverse outcomes were not observed when administering multiple doses of allogeneic BM-MSC.
Lay summary: Thermal injuries are a significant source of morbidity and mortality, constituting 5%-20% of all injuries and 4% of all deaths. Despite overall improvements in the management of acutely burned patients, morbidities associated with deeper burn injuries remain commonplace. Burn patients are too often left with significant tissue loss, scarring and contractions leading to physical loss of function and long-lasting psychological and emotional impacts.In previous studies, we have demonstrated the safety and efficacy of administering bone marrow-derived mesenchymal stem cells (BM-MSC) to chronic wounds with substantial improvement in healing and evidence of tissue regeneration. In this report, we have examined the application of BM-MSC to deep second-degree burn injuries in patients.The aim of the present phase I/II clinical trial was to examine the safety and efficacy of administering allogeneic BM-MSC to deep second-degree burns. We utilised two different dose levels at concentrations 2.5 × 103 and 5 × 103 cells/cm2. Patients with deep second-degree burn wounds up to 20% of the total body surface area were eligible for treatment. Allogeneic BM-MSC were applied to burn wounds topically or by injection under transparent film dressing <7 days after injury. Patients were followed for at least six months after treatment.Using two dose levels allowed us to gain preliminary information as to whether different amounts of BM-MSC administered to burn wounds will result in significant differences in safety/ clinical response. Once the safety and dose-response analysis were completed, we evaluated the efficacy of allogeneic stem cell therapy in the treatment of deep second-degree burn wounds.In this study, we examined the role of allogeneic BM-MSC treatment in patients with deep second-degree burn injuries, in a dose-dependent manner. No significant related adverse events were reported. Safety was evaluated both clinically and by laboratory-based methods. Efficacy was assessed clinically through evidence of re-pigmentation, hair follicle restoration and regenerative change. While these findings are encouraging, more studies will be needed to better establish the benefit of BM-MSC in the treatment of burn injuries.
Keywords: Second-degree burn; bone marrow–derived mesenchymal stem cells; burn scar; cell therapy; thermal injury; wound healing.
© The Author(s) 2022.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


Similar articles
-
[Clinical study of cell sheets containing allogeneic keratinocytes and fibroblasts for the treatment of partial-thickness burn wounds].Zhonghua Shao Shang Za Zhi. 2020 Mar 20;36(3):171-178. doi: 10.3760/cma.j.cn501120-20191113-00426. Zhonghua Shao Shang Za Zhi. 2020. PMID: 32241042 Chinese.
-
Treatment of superficial and deep partial width second degree burn's wound with allogeneic cord blood platelet gel.Skin Res Technol. 2023 Sep;29(9):e13471. doi: 10.1111/srt.13471. Skin Res Technol. 2023. PMID: 37753692 Free PMC article.
-
Role of cord blood and bone marrow mesenchymal stem cells in recent deep burn: a case-control prospective study.Am J Stem Cells. 2017 Oct 1;6(3):23-35. eCollection 2017. Am J Stem Cells. 2017. PMID: 29142785 Free PMC article.
-
Systematic Review of Stem-Cell-Based Therapy of Burn Wounds: Lessons Learned from Animal and Clinical Studies.Cells. 2020 Nov 26;9(12):2545. doi: 10.3390/cells9122545. Cells. 2020. PMID: 33256038 Free PMC article.
-
Application of Adipose-Tissue Derived Products for Burn Wound Healing.Pharmaceuticals (Basel). 2023 Sep 14;16(9):1302. doi: 10.3390/ph16091302. Pharmaceuticals (Basel). 2023. PMID: 37765109 Free PMC article. Review.
Cited by
-
Therapeutic Effects of Mesenchymal Stromal Cells Require Mitochondrial Transfer and Quality Control.Int J Mol Sci. 2023 Oct 31;24(21):15788. doi: 10.3390/ijms242115788. Int J Mol Sci. 2023. PMID: 37958771 Free PMC article. Review.
-
Advancements in cell-based therapies for thermal burn wounds: a comprehensive systematic review of clinical trials outcomes.Stem Cell Res Ther. 2024 Sep 4;15(1):277. doi: 10.1186/s13287-024-03901-2. Stem Cell Res Ther. 2024. PMID: 39227861 Free PMC article.
-
Effect of placental mesenchymal stem cells on promoting the healing of chronic burn wounds.Heliyon. 2024 Aug 22;10(17):e36584. doi: 10.1016/j.heliyon.2024.e36584. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39281490 Free PMC article.
-
Engineering strategies to enhance the research progress of mesenchymal stem cells in wound healing.Stem Cell Res Ther. 2025 Jul 1;16(1):342. doi: 10.1186/s13287-025-04471-7. Stem Cell Res Ther. 2025. PMID: 40598499 Free PMC article. Review.
-
Unveiling advanced strategies for therapeutic stem cell interventions in severe burn injuries: a comprehensive review.Int J Surg. 2024 Oct 1;110(10):6382-6401. doi: 10.1097/JS9.0000000000001812. Int J Surg. 2024. PMID: 38869979 Free PMC article. Review.
References
-
- Finkelstein E, Corso PS, Miller TR. The incidence and economic burden of injuries in the United States. Oxford; New York: Oxford University Press, 2006.
-
- Hudson DA, Renshaw A. An algorithm for the release of burn contractures of the extremities. Burns 2006; 32: 663–668. - PubMed
-
- Richard R, Baryza MJ, Carr JA, et al. Burn rehabilitation and research: proceedings of a consensus summit. J Burn Care Res 2009; 30: 543–573. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

