Augmented in vitro and in vivo Profiles of Brimonidine Tartrate Using Gelatinized-Core Liposomes
- PMID: 35782018
- PMCID: PMC9243147
- DOI: 10.2147/IJN.S370192
Augmented in vitro and in vivo Profiles of Brimonidine Tartrate Using Gelatinized-Core Liposomes
Abstract
Background: The low entrapment efficiency of the hydrophilic drugs such as brimonidine tartrate (BRT) in liposomes represents a challenge that requires interventions. Gelatinized core liposomes (GCLs) were fabricated to increase the drug entrapment, corneal penetration, and physical stability of the investigated molecule.
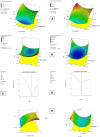
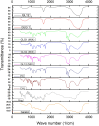
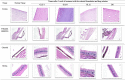
Research design and methods: GCLs encapsulating BRT were prepared and optimized utilizing D-optimal design (DOD). The effect of plasticizer incorporation on the physicochemical characteristics and on the in vivo performance was studied. The optimized formulations were investigated for pH, rheological properties, morphological characteristics, in vitro release profiles, biological performance, safety profile. The effects of storage and gamma sterilization were also investigated.
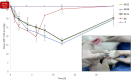
Results: The results revealed the great success of the prepared formulations to achieve high entrapment efficiency reaching 98% after a maturation period of 10 days. The addition of glycerol as plasticizer significantly minimized the particle size and shortened the maturation period to 7 days. The selected formulations were stable for 3 months after gamma sterilization. The formulations showed significant lowering of intra-ocular pressure (IOP) in glaucomatous rabbits with sustainment of the pharmacological effect for 24 hours compared to drug solution.
Conclusions: Enhanced in vitro and in vivo profiles of brimonidine tartrate loaded gelatinized-core-liposomes were obtained.
Keywords: BRT; D-optimal design; DOD; IOP; brimonidine tartrate; gelatin; gelatinized core liposomes; glaucoma; intra-ocular pressure; ocular drug delivery; plasticizer.
© 2022 Abdel Azim et al.
Conflict of interest statement
The authors declare no conflicts of interest in relation to this work.
Figures



Similar articles
-
Gelatinized core liposomes: A new Trojan horse for the development of a novel timolol maleate glaucoma medication.Int J Pharm. 2019 Feb 10;556:192-199. doi: 10.1016/j.ijpharm.2018.12.015. Epub 2018 Dec 12. Int J Pharm. 2019. PMID: 30553005
-
Cationic liposomes as promising vehicles for timolol/brimonidine combination ocular delivery in glaucoma: formulation development and in vitro/in vivo evaluation.Drug Deliv Transl Res. 2023 Apr;13(4):1035-1047. doi: 10.1007/s13346-022-01266-8. Epub 2022 Dec 7. Drug Deliv Transl Res. 2023. PMID: 36477776
-
Proniosomal gel-derived niosomes: an approach to sustain and improve the ocular delivery of brimonidine tartrate; formulation, in-vitro characterization, and in-vivo pharmacodynamic study.Drug Deliv. 2019 Dec;26(1):509-521. doi: 10.1080/10717544.2019.1609622. Drug Deliv. 2019. PMID: 31090464 Free PMC article.
-
Brimonidine tartrate for the treatment of glaucoma.Expert Opin Pharmacother. 2019 Jan;20(1):115-122. doi: 10.1080/14656566.2018.1544241. Epub 2018 Nov 8. Expert Opin Pharmacother. 2019. PMID: 30407890 Review.
-
The evolving pharmacotherapeutic profile of brimonidine, an alpha 2-adrenergic agonist, after four years of continuous use.Expert Opin Pharmacother. 2000 May;1(4):815-34. doi: 10.1517/14656566.1.4.815. Expert Opin Pharmacother. 2000. PMID: 11249518 Review.
Cited by
-
Physicochemical properties and micro-interaction between micro-nanoparticles and anterior corneal multilayer biological interface film for improving drug delivery efficacy: the transformation of tear film turnover mode.Drug Deliv. 2023 Dec;30(1):2184312. doi: 10.1080/10717544.2023.2184312. Drug Deliv. 2023. PMID: 36866574 Free PMC article.
-
Recent Advances of Ocular Drug Delivery Systems: Prominence of Ocular Implants for Chronic Eye Diseases.Pharmaceutics. 2023 Jun 15;15(6):1746. doi: 10.3390/pharmaceutics15061746. Pharmaceutics. 2023. PMID: 37376194 Free PMC article. Review.
-
Therapeutic targeting of ocular diseases with emphasis on PI3K/Akt, and OPRL pathways by Hedera helix L. saponins: a new approach for the treatment of Pseudomonas aeruginosa-induced bacterial keratitis.Nat Prod Bioprospect. 2025 May 12;15(1):30. doi: 10.1007/s13659-025-00514-x. Nat Prod Bioprospect. 2025. PMID: 40353954 Free PMC article.
-
Self-Healing Alginate Hydrogel Formed by Dynamic Benzoxaborolate Chemistry Protects Retinal Pigment Epithelium Cells against Oxidative Damage.Gels. 2022 Dec 29;9(1):24. doi: 10.3390/gels9010024. Gels. 2022. PMID: 36661792 Free PMC article.
References
-
- Cholkar K, Dasari SR, Pal D, Mitra AK. Eye: anatomy, physiology and barriers to drug delivery. In: Ocular Transporters and Receptors. Elsevier; 2013:1–36.
-
- Prakash P, Gnanaprakasam P, Emmanuel R, Arokiyaraj S, Saravanan M. Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf B Biointerfaces. 2013;108:255–259. doi:10.1016/j.colsurfb.2013.03.017 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

