Direct Visualization of Supramolecular Binding and Separation of Light Hydrocarbons in MFM-300(In)
- PMID: 35782207
- PMCID: PMC9245183
- DOI: 10.1021/acs.chemmater.2c01097
Direct Visualization of Supramolecular Binding and Separation of Light Hydrocarbons in MFM-300(In)
Abstract
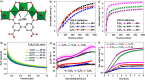
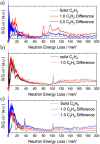
The purification of light olefins is one of the most important chemical separations globally and consumes large amounts of energy. Porous materials have the capability to improve the efficiency of this process by acting as solid, regenerable adsorbents. However, to develop translational systems, the underlying mechanisms of adsorption in porous materials must be fully understood. Herein, we report the adsorption and dynamic separation of C2 and C3 hydrocarbons in the metal-organic framework MFM-300(In), which exhibits excellent performance in the separation of mixtures of ethane/ethylene and propyne/propylene. Unusually selective adsorption of ethane over ethylene at low pressure is observed, resulting in selective retention of ethane from a mixture of ethylene/ethane, thus demonstrating its potential for a one-step purification of ethylene (purity > 99.9%). In situ neutron powder diffraction and inelastic neutron scattering reveal the preferred adsorption domains and host-guest binding dynamics of adsorption of C2 and C3 hydrocarbons in MFM-300(In).
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Theoretical investigation of C1-C4 hydrocarbons adsorption and separation in a porous metallocavitand.RSC Adv. 2022 Nov 29;12(52):34053-34065. doi: 10.1039/d2ra07183e. eCollection 2022 Nov 22. RSC Adv. 2022. PMID: 36544998 Free PMC article.
-
Purification of Propylene and Ethylene by a Robust Metal-Organic Framework Mediated by Host-Guest Interactions.Angew Chem Int Ed Engl. 2021 Jul 5;60(28):15541-15547. doi: 10.1002/anie.202103936. Epub 2021 Jun 7. Angew Chem Int Ed Engl. 2021. PMID: 33826198 Free PMC article.
-
Supramolecular binding and separation of hydrocarbons within a functionalized porous metal-organic framework.Nat Chem. 2014 Feb;7(2):121-9. doi: 10.1038/nchem.2114. Epub 2014 Dec 1. Nat Chem. 2014. PMID: 25615665
-
Light Hydrocarbon Separations Using Porous Organic Framework Materials.Chemistry. 2020 Mar 12;26(15):3205-3221. doi: 10.1002/chem.201904455. Epub 2019 Dec 27. Chemistry. 2020. PMID: 31667891 Review.
-
Metal-Organic Framework Materials for the Separation and Purification of Light Hydrocarbons.Adv Mater. 2020 Jan;32(3):e1806445. doi: 10.1002/adma.201806445. Epub 2019 May 20. Adv Mater. 2020. PMID: 31106907 Review.
Cited by
-
Theoretical investigation of C1-C4 hydrocarbons adsorption and separation in a porous metallocavitand.RSC Adv. 2022 Nov 29;12(52):34053-34065. doi: 10.1039/d2ra07183e. eCollection 2022 Nov 22. RSC Adv. 2022. PMID: 36544998 Free PMC article.
-
Highly Productive C3H4/C3H6 Trace Separation by a Packing Polymorph of a Layered Hybrid Ultramicroporous Material.J Am Chem Soc. 2023 May 31;145(21):11837-11845. doi: 10.1021/jacs.3c03505. Epub 2023 May 19. J Am Chem Soc. 2023. PMID: 37204941 Free PMC article.
References
-
- Blay V.; Louis B.; Miravalles R.; Yokoi T.; Peccatiello K. A.; Clough M.; Yilmaz B. Engineering Zeolites for Catalytic Cracking to Light Olefins. ACS Catal. 2017, 7, 6542–6566. 10.1021/acscatal.7b02011. - DOI
-
- Ren T.; Patel M.; Blok K. Olefins from Conventional and Heavy Feedstocks: Energy Use in Steam Cracking and Alternative Processes. Energy 2006, 31, 425–451. 10.1016/j.energy.2005.04.001. - DOI
-
- Geng S.; Lin E.; Li X.; Liu W.; Wang T.; Wang Z.; Sensharma D.; Darwish S.; Andaloussi Y. H.; Pham T.; Cheng P.; Zaworotko M. J.; Chen Y.; Zhang Z. Scalable Room-Temperature Synthesis of Highly Robust Ethane-Selective Metal-Organic Frameworks for Efficient Ethylene Purification. J. Am. Chem. Soc. 2021, 143, 8654–8660. 10.1021/jacs.1c02108. - DOI - PubMed
-
- Yang R. T.; Kikkinides E. S. New Sorbents for Olefin/Paraffin Separations by Adsorption via π-Complexation. AIChE J. 1995, 41, 509–517. 10.1002/aic.690410309. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
