Hydrogels for Single-Cell Microgel Production: Recent Advances and Applications
- PMID: 35782502
- PMCID: PMC9247248
- DOI: 10.3389/fbioe.2022.891461
Hydrogels for Single-Cell Microgel Production: Recent Advances and Applications
Abstract
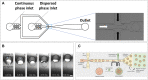
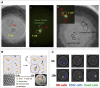
Single-cell techniques have become more and more incorporated in cell biological research over the past decades. Various approaches have been proposed to isolate, culture, sort, and analyze individual cells to understand cellular heterogeneity, which is at the foundation of every systematic cellular response in the human body. Microfluidics is undoubtedly the most suitable method of manipulating cells, due to its small scale, high degree of control, and gentle nature toward vulnerable cells. More specifically, the technique of microfluidic droplet production has proven to provide reproducible single-cell encapsulation with high throughput. Various in-droplet applications have been explored, ranging from immunoassays, cytotoxicity assays, and single-cell sequencing. All rely on the theoretically unlimited throughput that can be achieved and the monodispersity of each individual droplet. To make these platforms more suitable for adherent cells or to maintain spatial control after de-emulsification, hydrogels can be included during droplet production to obtain "microgels." Over the past years, a multitude of research has focused on the possibilities these can provide. Also, as the technique matures, it is becoming clear that it will result in advantages over conventional droplet approaches. In this review, we provide a comprehensive overview on how various types of hydrogels can be incorporated into different droplet-based approaches and provide novel and more robust analytic and screening applications. We will further focus on a wide range of recently published applications for microgels and how these can be applied in cell biological research at the single- to multicell scale.
Keywords: droplet; hydrogel; immunology; microfluidics; microgel; pairing; single cell.
Copyright © 2022 Tiemeijer and Tel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Hydrogel Droplet Microfluidics for High-Throughput Single Molecule/Cell Analysis.Acc Chem Res. 2017 Jan 17;50(1):22-31. doi: 10.1021/acs.accounts.6b00370. Epub 2016 Dec 28. Acc Chem Res. 2017. PMID: 28029779
-
In-air production of 3D co-culture tumor spheroid hydrogels for expedited drug screening.Acta Biomater. 2019 Aug;94:392-409. doi: 10.1016/j.actbio.2019.06.012. Epub 2019 Jun 12. Acta Biomater. 2019. PMID: 31200118
-
Single-cell droplet microfluidics for biomedical applications.Analyst. 2022 May 30;147(11):2294-2316. doi: 10.1039/d1an02321g. Analyst. 2022. PMID: 35506869 Review.
-
Massive and efficient encapsulation of single cells in monodisperse droplets and collagen-alginate microgels using a microfluidic device.Front Bioeng Biotechnol. 2023 Nov 15;11:1281375. doi: 10.3389/fbioe.2023.1281375. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 38033813 Free PMC article.
-
Trends in Droplet Microfluidics: From Droplet Generation to Biomedical Applications.Langmuir. 2022 May 24;38(20):6233-6248. doi: 10.1021/acs.langmuir.2c00491. Epub 2022 May 13. Langmuir. 2022. PMID: 35561292 Review.
Cited by
-
Bone/cartilage targeted hydrogel: Strategies and applications.Bioact Mater. 2022 Nov 11;23:156-169. doi: 10.1016/j.bioactmat.2022.10.028. eCollection 2023 May. Bioact Mater. 2022. PMID: 36406248 Free PMC article. Review.
-
Mechanoactivation of Single Stem Cells in Microgels Using a 3D-Printed Stimulation Device.Small Methods. 2024 Dec;8(12):e2400272. doi: 10.1002/smtd.202400272. Epub 2024 Jul 16. Small Methods. 2024. PMID: 39011729 Free PMC article.
-
Single-cell analysis reveals TLR-induced macrophage heterogeneity and quorum sensing dictate population wide anti-inflammatory feedback in response to LPS.Front Immunol. 2023 Feb 24;14:1135223. doi: 10.3389/fimmu.2023.1135223. eCollection 2023. Front Immunol. 2023. PMID: 36911668 Free PMC article.
-
A Microfluidic Approach for Probing Heterogeneity in Cytotoxic T-Cells by Cell Pairing in Hydrogel Droplets.Micromachines (Basel). 2022 Nov 4;13(11):1910. doi: 10.3390/mi13111910. Micromachines (Basel). 2022. PMID: 36363930 Free PMC article.
-
Minimally Invasive Syringe-Injectable Hydrogel with Angiogenic Factors for Ischemic Stroke Treatment.Adv Healthc Mater. 2025 Mar;14(6):e2403119. doi: 10.1002/adhm.202403119. Epub 2024 Nov 9. Adv Healthc Mater. 2025. PMID: 39520382 Free PMC article.
References
-
- Antona S., Abele T., Jahnke K., Yannik D., Kerstin G., Ilia P. (2020a). Droplet-Based Combinatorial Assay for Cell Cytotoxicity and Cytokine Release Evaluation. Adv. Funct. Mater. 30 (46). 10.1002/adfm.202003479 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

