Septotemporal variation in modulation of synaptic transmission, paired-pulse ratio and frequency facilitation/depression by adenosine and GABAB receptors in the rat hippocampus
- PMID: 35782711
- PMCID: PMC9240614
- DOI: 10.1177/23982128221106315
Septotemporal variation in modulation of synaptic transmission, paired-pulse ratio and frequency facilitation/depression by adenosine and GABAB receptors in the rat hippocampus
Abstract
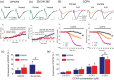
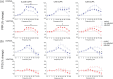
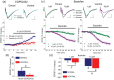
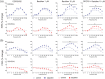
Short-term synaptic plasticity represents a fundamental mechanism in neural information processing and is regulated by neuromodulators. Here, using field recordings from the CA1 region of adult rat hippocampal slices, we show that excitatory synaptic transmission is suppressed by strong but not moderate activation of adenosine A1 receptors by 2-Chloro-N6-cyclopentyladenosine (CCPA) more in the dorsal than the ventral hippocampus; in contrast, both mild and strong activation of GABAB receptors by baclofen (1 μM, 10 μM) suppress synaptic transmission more in the ventral than the dorsal hippocampus. Using a 10-pulse stimulation train of variable frequency, we found that CCPA modulates short-term synaptic plasticity independently of the suppression of synaptic transmission in both segments of the hippocampus and at stimulation frequencies greater than 10 Hz. However, specifically regarding the paired-pulse ratio (PPR) and frequency facilitation/depression (FF/D) we found significant drug action before but not after adjusting conditioning responses to control levels. Activation of GABABRs by baclofen suppressed synaptic transmission more in the ventral than the dorsal hippocampus. Furthermore, relatively high (10 μM) but not low (1 μM) baclofen concentration enhanced both PPR and FF in both hippocampal segments at stimulation frequencies greater than 1 Hz, independently of the suppression of synaptic transmission by baclofen. These results show that A1Rs and GABABRs control synaptic transmission more effectively in the dorsal and the ventral hippocampus, respectively, and suggest that these receptors modulate PPR and FF/D at different frequency bands of afferent input, in both segments of the hippocampus.
Keywords: GABAb receptors; Hippocampus; adenosine receptors; dorsoventral; in vitro; longitudinal axis; neuromodulation; rat; septotemporal; short-term synaptic plasticity.
© The Author(s) 2022.
Conflict of interest statement
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures



Similar articles
-
Dorsal-Ventral Differences in Modulation of Synaptic Transmission in the Hippocampus.Front Synaptic Neurosci. 2020 Jun 18;12:24. doi: 10.3389/fnsyn.2020.00024. eCollection 2020. Front Synaptic Neurosci. 2020. PMID: 32625076 Free PMC article.
-
Short-term dynamics of input and output of CA1 network greatly differ between the dorsal and ventral rat hippocampus.BMC Neurosci. 2019 Jul 22;20(1):35. doi: 10.1186/s12868-019-0517-5. BMC Neurosci. 2019. PMID: 31331291 Free PMC article.
-
Muscarinic Modulation of Synaptic Transmission and Short-Term Plasticity in the Dorsal and Ventral Hippocampus.Mol Cell Neurosci. 2024 Jun;129:103935. doi: 10.1016/j.mcn.2024.103935. Epub 2024 May 3. Mol Cell Neurosci. 2024. PMID: 38703973
-
A gradient of frequency-dependent synaptic properties along the longitudinal hippocampal axis.BMC Neurosci. 2017 Dec 12;18(1):79. doi: 10.1186/s12868-017-0398-4. BMC Neurosci. 2017. PMID: 29233091 Free PMC article.
-
Striking differences in synaptic facilitation along the dorsoventral axis of the hippocampus.Neuroscience. 2015 Aug 20;301:454-70. doi: 10.1016/j.neuroscience.2015.06.029. Epub 2015 Jun 23. Neuroscience. 2015. PMID: 26116517
Cited by
-
Region-Related Differences in Short-Term Synaptic Plasticity and Synaptotagmin-7 in the Male and Female Hippocampus of a Rat Model of Fragile X Syndrome.Int J Mol Sci. 2024 Jun 26;25(13):6975. doi: 10.3390/ijms25136975. Int J Mol Sci. 2024. PMID: 39000085 Free PMC article.
-
Incremental induction of NMDAR-STP and NMDAR-LTP in the CA1 area of ventral hippocampal slices relies on graded activation of discrete NMDA receptors.Philos Trans R Soc Lond B Biol Sci. 2024 Jul 29;379(1906):20230239. doi: 10.1098/rstb.2023.0239. Epub 2024 Jun 10. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 38853568 Free PMC article.
References
-
- Abbott LF, Regehr WG. (2004) Synaptic computation. Nature 431(7010): 796–803. - PubMed
-
- Abbott LF, Varela JA, Sen K, et al.. (1997) Synaptic depression and cortical gain control. Science 275(5297): 220–224. - PubMed
-
- Bannerman DM, Deacon RM, Offen S, et al.. (2002) Double dissociation of function within the hippocampus: Spatial memory and hyponeophagia. Behavioral Neuroscience 116(5): 884–901. - PubMed
-
- Bannerman DM, Sprengel R, Sanderson DJ, et al.. (2014) Hippocampal synaptic plasticity, spatial memory and anxiety. Nature Reviews. Neuroscience 15(3): 181–192. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

