Evolving landscape of first-line combination therapy in advanced renal cancer: a systematic review
- PMID: 35782749
- PMCID: PMC9244935
- DOI: 10.1177/17588359221108685
Evolving landscape of first-line combination therapy in advanced renal cancer: a systematic review
Abstract
Background: Renal cell carcinoma (RCC) is a common malignancy with approximately 30% of cases diagnosed at the advanced or metastatic stage. While single-agent vascular endothelial growth factor-targeted therapy has been a mainstay of treatment, data from multiple phase III trials assessing first-line immune checkpoint inhibitor (ICI) combinations have demonstrated a significant survival benefit.
Methods: A systematic search of the published and presented literature was performed to identify phase III trials assessing ICI combination regimens in RCC using search terms 'immune checkpoint inhibitors' AND 'renal cell carcinoma,' AND 'advanced'.
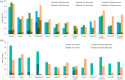
Results: Six phase III trials showed significant benefits for ICI combinations compared with sunitinib. Nivolumab plus ipilimumab significantly improved overall survival [OS; median, 47.0 versus 26.6 months, hazard ratio (HR) = 0.68, 95% confidence interval (CI) = 0.58-0.81, p < 0.0001) and progression-free survival (PFS; median 11.6 versus 8.3 months, HR = 0.73, 95% CI = 0.61-0.87, p = 0.0004) in International Metastatic renal cell carcinoma Database Consortium intermediate and poor-risk patients. OS was also significantly improved for ICI plus tyrosine kinase inhibitor combinations regardless of risk, including pembrolizumab plus either axitinib (HR = 0.73, 95% CI = 0.60-0.88, p < 0.001) or lenvatinib (HR = 0.66, 95% CI = 0.49-0.88, p = 0.005) and nivolumab plus cabozantinib (HR = 0.66, 95% CI = 0.50-0.87, p = 0.003). No new safety signals were identified.
Conclusions: Phase III first-line trials of ICI combinations showed survival benefits compared with a control arm of sunitinib. Global access to these combinations should be made available to patients with advanced RCC.
Keywords: advanced disease; combination therapy; immune checkpoint inhibitors; metastatic or locally targeted therapy; renal carcinoma; tyrosine kinase inhibitors.
© The Author(s), 2022.
Figures


References
-
- Sung H, Ferlay J, Siegel RL, et al.. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. - PubMed
-
- Jacobsen J, Grankvist K, Rasmuson T, et al.. Expression of vascular endothelial growth factor protein in human renal cell carcinoma. BJU Int 2004; 93: 297–302. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

