Major Aortopulmonary Collateral Arteries
- PMID: 35782757
- PMCID: PMC8893210
- DOI: 10.1148/ryct.210157
Major Aortopulmonary Collateral Arteries
Abstract
Major aortopulmonary collateral arteries (MAPCAs) are congenital vessels that arise from the aorta or its first-order branches and are distally connected to the pulmonary arterial vasculature, thereby providing pulmonary blood flow. MAPCAs are commonly associated with several congenital heart diseases that have compromised pulmonary circulation due to severe stenosis involving pulmonary valves or arteries or due to pulmonary atresia. Embryologically, MAPCAs are presumed to be persistent segmental arteries. MAPCAs can be imaged with CT and MRI, and such imaging findings are important for surgeons and interventionists. The management options for MAPCAs include unifocalization, surgical ligation, and endovascular interventions, such as coil embolization. This review highlights the role of reporting certain critical features of MAPCAs at CT and MRI, which will help to facilitate management decisions for systemic-to-pulmonary collateral vessels observed in patients with congenital heart disease. Keywords: Pediatrics, CT Angiography, Image Postprocessing, Interventional-Vascular, MR Angiography, Embolization, Stents, Cardiac, Vascular, Aorta © RSNA, 2022.
Keywords: Aorta; CT Angiography; Cardiac; Embolization; Image Postprocessing; Interventional-Vascular; MR Angiography; Pediatrics; Stents; Vascular.
2022 by the Radiological Society of North America, Inc.
Conflict of interest statement
Disclosures of Conflicts of Interest: A. Alex No relevant relationships. A. Ayyappan No relevant relationships. J.V. No relevant relationships. H.K. No relevant relationships. D.S. No relevant relationships. S.M. No relevant relationships.
Figures








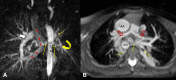



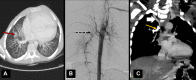
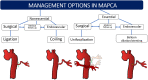
References
-
- Wernovsky G , Anderson RH , Kumar K , et al. , eds. Anderson’s Pediatric Cardiology . 4th ed. Philadelphia, Pa: : Elsevier; , 2019. ; 660 – 663 .
-
- Marino B , Calabró R , Gagliardi MG , Bevilacqua M , Ballerini L , Marcelletti C . Patterns of pulmonary arterial anatomy and blood supply in complex congenital heart disease with pulmonary atresia . J Thorac Cardiovasc Surg 1987. ; 94 ( 4 ): 518 – 520 . - PubMed
-
- Ryan JR , Moe TG , Richardson R , Frakes DH , Nigro JJ , Pophal S . A novel approach to neonatal management of tetralogy of Fallot, with pulmonary atresia, and multiple aortopulmonary collaterals . JACC Cardiovasc Imaging 2015. ; 8 ( 1 ): 103 – 104 . - PubMed
-
- Seale AN , Ho SY , Shinebourne EA , Carvalho JS . Prenatal identification of the pulmonary arterial supply in tetralogy of Fallot with pulmonary atresia . Cardiol Young 2009. ; 19 ( 2 ): 185 – 191 . - PubMed
Publication types
LinkOut - more resources
Full Text Sources

