Platelet-to-lymphocyte and neutrophil-to-lymphocyte ratios are associated with the efficacy of immunotherapy in stage III/IV non-small cell lung cancer
- PMID: 35782904
- PMCID: PMC9247654
- DOI: 10.3892/ol.2022.13386
Platelet-to-lymphocyte and neutrophil-to-lymphocyte ratios are associated with the efficacy of immunotherapy in stage III/IV non-small cell lung cancer
Abstract
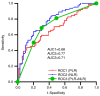
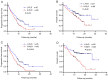
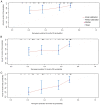
Peripheral serological indicators are novel markers associated with prognosis in multiple malignant tumors. In the present study, platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR) were selected to construct a model that predicts long-term survival of patients with stage IIIB-IV non-small cell lung cancer (NSCLC) who received treatment with an anti-programmed cell death protein-1 (PD-1) monoclonal antibody. A total of 133 patients were eligible for the present retrospective study (January 2019-February 2021). The area under the receiver operating characteristic curve was used to compare the diagnostic value of PLR and NLR, and combined PLR and NLR. The objective response rate and disease control rate of each group were obtained and the differences were compared using the χ2 test. The prognostic value of these indicators was assessed using the Kaplan-Meier method. Cox regression analysis was used to evaluate risk factors associated with long-term survival. Statistically significant parameters were included in the nomogram. Based on the median PLR and NLR values, the patients were divided into high PLR (H-PLR) (PLR >200.00, 67 patients) and low PLR (L-PLR) (PLR ≤200.00, 66 patients), and high NLR (H-NLR) (NLR >3.56, 65 patients) and low NLR (L-NLR) (NLR ≤3.56, 68 patients) groups. Immune-related adverse events (irAEs) occurred in 22 patients (16.5%) during the observation period, including 18 grade 2-3 irAEs and 4 grade 4 cases. H-NLR and H-PLR were associated with poor progression-free (PFS) and overall survival (OS) in the present study. NLR was an independent prognostic factor for PFS [hazard ratio (HR): 0.201, 95% confidence interval (CI): 0.060-0.670; P=0.009) and OS (HR: 0.413, 95% CI: 0.226-0.754; P=0.004) in this patient group. Therefore, NLR may be used in the prognostication of patients with stage IIIB-IV NSCLC treated with PD-1 inhibitors. These serological markers may be used in combination with established immunomarkers to help predict outcomes.
Keywords: NLR; NSCLC; PD-1; PD-L1; PLR; immunotherapy; inflammatory indicators.
Copyright: © Lu et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Duan J, Cui L, Zhao X, Bai H, Cai S, Wang G, Zhao Z, Zhao J, Chen S, Song J, et al. Use of immunotherapy with programmed cell death 1 vs programmed cell death ligand 1 inhibitors in patients with cancer: A systematic review and meta-analysis. JAMA Oncol. 2020;6:375–384. doi: 10.1001/jamaoncol.2019.5367. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
