Clinical translation of a patient-specific scaffold-guided bone regeneration concept in four cases with large long bone defects
- PMID: 35782964
- PMCID: PMC9213234
- DOI: 10.1016/j.jot.2022.04.004
Clinical translation of a patient-specific scaffold-guided bone regeneration concept in four cases with large long bone defects
Abstract
Background: Bone defects after trauma, infection, or tumour resection present a challenge for patients and clinicians. To date, autologous bone graft (ABG) is the gold standard for bone regeneration. To address the limitations of ABG such as limited harvest volume as well as overly fast remodelling and resorption, a new treatment strategy of scaffold-guided bone regeneration (SGBR) was developed. In a well-characterized sheep model of large to extra-large tibial segmental defects, three-dimensional (3D) printed composite scaffolds have shown clinically relevant biocompatibility and osteoconductive capacity in SGBR strategies. Here, we report four challenging clinical cases with large complex posttraumatic long bone defects using patient-specific SGBR as a successful treatment.
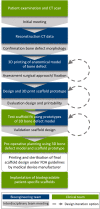
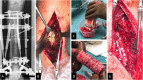
Methods: After giving informed consent computed tomography (CT) images were used to design patient-specific biodegradable medical-grade polycaprolactone-tricalcium phosphate (mPCL-TCP, 80:20 wt%) scaffolds. The CT scans were segmented using Materialise Mimics to produce a defect model and the scaffold parts were designed with Autodesk Meshmixer. Scaffold prototypes were 3D-printed to validate robust clinical handling and bone defect fit. The final scaffold design was additively manufactured under Food and Drug Administration (FDA) guidelines for patient-specific and custom-made implants by Osteopore International Pte Ltd.
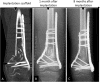
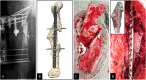
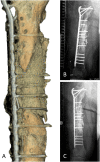
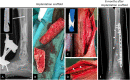
Results: Four patients (age: 23-42 years) with posttraumatic lower extremity large long bone defects (case 1: 4 cm distal femur, case 2: 10 cm tibia shaft, case 3: complex malunion femur, case 4: irregularly shaped defect distal tibia) are presented. After giving informed consent, the patients were treated surgically by implanting a custom-made mPCL-TCP scaffold loaded with ABG (case 2: additional application of recombinant human bone morphogenetic protein-2) harvested with the Reamer-Irrigator-Aspirator system (RIA, Synthes®). In all cases, the scaffolds matched the actual anatomical defect well and no perioperative adverse events were observed. Cases 1, 3 and 4 showed evidence of bony ingrowth into the large honeycomb pores (pores >2 mm) and fully interconnected scaffold architecture with indicative osseous bridges at the bony ends on the last radiographic follow-up (8-9 months after implantation). Comprehensive bone regeneration and full weight bearing were achieved in case 2 at follow-up 23 months after implantation.
Conclusion: This study shows the bench to bedside translation of guided bone regeneration principles into scaffold-based bone tissue engineering. The scaffold design in SGBR should have a tissue-specific morphological signature which stimulates and directs the stages from the initial host response towards the full regeneration. Thereby, the scaffolds provide a physical niche with morphology and biomaterial properties that allow cell migration, proliferation, and formation of vascularized tissue in the first one to two months, followed by functional bone formation and the capacity for physiological bone remodelling. Great design flexibility of composite scaffolds to support the one to three-year bone regeneration was observed in four patients with complex long bone defects.
The translational potential of this article: This study reports on the clinical efficacy of SGBR in the treatment of long bone defects. Moreover, it presents a comprehensive narrative of the rationale of this technology, highlighting its potential for bone regeneration treatment regimens in patients with any type of large and complex osseous defects.
Keywords: additive manufacturing; bone; defect; non-union; polycaprolactone; scaffold.
Crown Copyright © 2022 Published by Elsevier (Singapore) Pte Ltd on behalf of Chinese Speaking Orthopaedic Society.
Conflict of interest statement
DWH is a cofounder and shareholder of Osteopore International Pty Ltd., a company specialized in 3D-printed bioresorbable implants to assist with bone healing. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
In vivo characterization of 3D-printed polycaprolactone-hydroxyapatite scaffolds with Voronoi design to advance the concept of scaffold-guided bone regeneration.Front Bioeng Biotechnol. 2023 Oct 4;11:1272348. doi: 10.3389/fbioe.2023.1272348. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37860627 Free PMC article.
-
The Concept of Scaffold-Guided Bone Regeneration for the Treatment of Long Bone Defects: Current Clinical Application and Future Perspective.J Funct Biomater. 2023 Jun 27;14(7):341. doi: 10.3390/jfb14070341. J Funct Biomater. 2023. PMID: 37504836 Free PMC article. Review.
-
Convergence of scaffold-guided bone regeneration and RIA bone grafting for the treatment of a critical-sized bone defect of the femoral shaft.Eur J Med Res. 2020 Dec 21;25(1):70. doi: 10.1186/s40001-020-00471-w. Eur J Med Res. 2020. PMID: 33349266 Free PMC article.
-
An innovative intramedullary bone graft harvesting concept as a fundamental component of scaffold-guided bone regeneration: A preclinical in vivo validation.J Orthop Translat. 2024 Jun 5;47:1-14. doi: 10.1016/j.jot.2024.05.002. eCollection 2024 Jul. J Orthop Translat. 2024. PMID: 38957270 Free PMC article.
-
Biodegradable interbody cages for lumbar spine fusion: Current concepts and future directions.Biomaterials. 2022 Sep;288:121699. doi: 10.1016/j.biomaterials.2022.121699. Epub 2022 Aug 8. Biomaterials. 2022. PMID: 35995620 Review.
Cited by
-
Injectable ultrasound-powered bone-adhesive nanocomposite hydrogel for electrically accelerated irregular bone defect healing.J Nanobiotechnology. 2024 Feb 7;22(1):54. doi: 10.1186/s12951-024-02320-y. J Nanobiotechnology. 2024. PMID: 38326903 Free PMC article.
-
In vivo characterization of 3D-printed polycaprolactone-hydroxyapatite scaffolds with Voronoi design to advance the concept of scaffold-guided bone regeneration.Front Bioeng Biotechnol. 2023 Oct 4;11:1272348. doi: 10.3389/fbioe.2023.1272348. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37860627 Free PMC article.
-
Recent Methods for Modifying Mechanical Properties of Tissue-Engineered Scaffolds for Clinical Applications.Biomimetics (Basel). 2023 May 16;8(2):205. doi: 10.3390/biomimetics8020205. Biomimetics (Basel). 2023. PMID: 37218791 Free PMC article. Review.
-
The Concept of Scaffold-Guided Bone Regeneration for the Treatment of Long Bone Defects: Current Clinical Application and Future Perspective.J Funct Biomater. 2023 Jun 27;14(7):341. doi: 10.3390/jfb14070341. J Funct Biomater. 2023. PMID: 37504836 Free PMC article. Review.
-
Faster Bone Gap Union in Medial Opening Wedge High Tibial Osteotomy With 3D-Printed Synthetic Bioresorbable Polycaprolactone and Tricalcium Phosphate Osteotomy Gap Fillers Compared to Allogeneic Osteotomy Gap Fillers: A Retrospective Matched-Pair Cohort Study.Cartilage. 2025 Mar;16(1):24-35. doi: 10.1177/19476035241246609. Epub 2024 Apr 16. Cartilage. 2025. PMID: 38624072 Free PMC article.
References
-
- Hadjidakis D.J., Androulakis I.I. Bone remodeling. Ann N Y Acad Sci. 2006;1092:385–396. [eng] - PubMed
-
- Hak D.J., Fitzpatrick D., Bishop J.A., Marsh J.L., Tilp S., Schnettler R., et al. Delayed union and nonunions: epidemiology, clinical issues, and financial aspects. Injury. 2014;45(Suppl 2):S3–S7. [eng] - PubMed
-
- Lerner R.K., Esterhai J.L., Jr., Polomano R.C., Cheatle M.D., Heppenstall R.B. Quality of life assessment of patients with posttraumatic fracture nonunion, chronic refractory osteomyelitis, and lower-extremity amputation. Clin Orthop Relat Res. 1993;295:28–36. [eng] - PubMed
-
- Zhao D., Witte F., Lu F., Wang J., Li J., Qin L. Current status on clinical applications of magnesium-based orthopaedic implants: a review from clinical translational perspective. Biomaterials. 2017;112:287–302. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

