Porphyrin-Based Multicomponent Metallacage: Host-Guest Complexation toward Photooxidation-Triggered Reversible Encapsulation and Release
- PMID: 35783178
- PMCID: PMC9241011
- DOI: 10.1021/jacsau.2c00245
Porphyrin-Based Multicomponent Metallacage: Host-Guest Complexation toward Photooxidation-Triggered Reversible Encapsulation and Release
Abstract
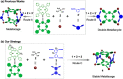
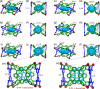
The development of supramolecular hosts with effective host-guest properties is crucial for their applications. Herein, we report the preparation of a porphyrin-based metallacage, which serves as a host for a series of polycyclic aromatic hydrocarbons (PAHs). The association constant between the metallacage and coronene reaches 2.37 × 107 M-1 in acetonitrile/chloroform (ν/ν = 9/1), which is among the highest values in metallacage-based host-guest complexes. Moreover, the metallacage exhibits good singlet oxygen generation capacity, which can be further used to oxidize encapsulated anthracene derivatives into anthracene endoperoxides, leading to the release of guests. By employing 10-phenyl-9-(2-phenylethynyl)anthracene whose endoperoxide can be converted back by heating as the guest, a reversible controlled release system is constructed. This study not only gives a type of porphyrin-based metallacage that shows desired host-guest interactions with PAHs but also offers a photooxidation-responsive host-guest recognition motif, which will guide future design and applications of metallacages for stimuli-responsive materials.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Enhancing the Photosensitivity of Hypocrellin A by Perylene Diimide Metallacage-Based Host-Guest Complexation for Photodynamic Therapy.Nanomicro Lett. 2024 Jun 25;16(1):226. doi: 10.1007/s40820-024-01438-w. Nanomicro Lett. 2024. PMID: 38916749 Free PMC article.
-
Guest-Regulated Generation of Reactive Oxygen Species from Porphyrin-Based Multicomponent Metallacages for Selective Photocatalysis.Angew Chem Int Ed Engl. 2024 Apr 2;63(14):e202319488. doi: 10.1002/anie.202319488. Epub 2024 Feb 22. Angew Chem Int Ed Engl. 2024. PMID: 38305830
-
Multicomponent, Multicavity Metallacages That Contain Different Binding Sites for Allosteric Recognition.J Am Chem Soc. 2024 Jun 12;146(23):15787-15795. doi: 10.1021/jacs.4c01873. Epub 2024 May 13. J Am Chem Soc. 2024. PMID: 38738985
-
Noncovalent tailoring of coordination complexes by resorcin[4]arene-based supramolecular hosts.Dalton Trans. 2023 May 22;52(20):6604-6618. doi: 10.1039/d3dt00710c. Dalton Trans. 2023. PMID: 37128873 Review.
-
Supramolecular chemistry of p-sulfonatocalix[n]arenes and its biological applications.Acc Chem Res. 2014 Jul 15;47(7):1925-34. doi: 10.1021/ar500009g. Epub 2014 Mar 25. Acc Chem Res. 2014. PMID: 24666259 Review.
Cited by
-
Engineering cofacial porphyrin dimers using lacunary polyoxotungstates.Chem Sci. 2025 Apr 22;16(21):9178-9185. doi: 10.1039/d5sc00814j. eCollection 2025 May 28. Chem Sci. 2025. PMID: 40303458 Free PMC article.
-
Dynamic selection in metallo-organic cube CdII 8L4 conformations induced by perfluorooctanoate encapsulation.Chem Sci. 2024 Nov 22;16(1):364-370. doi: 10.1039/d4sc07105k. eCollection 2024 Dec 18. Chem Sci. 2024. PMID: 39620083 Free PMC article.
-
Enhancing the Photosensitivity of Hypocrellin A by Perylene Diimide Metallacage-Based Host-Guest Complexation for Photodynamic Therapy.Nanomicro Lett. 2024 Jun 25;16(1):226. doi: 10.1007/s40820-024-01438-w. Nanomicro Lett. 2024. PMID: 38916749 Free PMC article.
-
Capture of Singlet Oxygen Modulates Host-Guest Behavior of Coordination Cages.Angew Chem Int Ed Engl. 2023 Sep 25;62(39):e202309589. doi: 10.1002/anie.202309589. Epub 2023 Aug 23. Angew Chem Int Ed Engl. 2023. PMID: 37610599 Free PMC article.
-
Fusion of two homoleptic truncated tetrahedra into a heteroleptic truncated octahedron.Chem Sci. 2024 Aug 9;15(35):14352-7. doi: 10.1039/d4sc02736a. Online ahead of print. Chem Sci. 2024. PMID: 39165732 Free PMC article.
References
-
- Pedersen C. J. Cyclic Polyethers and Their Complexes with Metal Salts. J. Am. Chem. Soc. 1967, 89, 7017–7036. 10.1021/ja01002a035. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
