Reduction Kinetic of Water Soluble Metal Salts by Geobacter sulfurreducens: Fe2+/Hemes Stabilize and Regulate Electron Flux Rates
- PMID: 35783399
- PMCID: PMC9248073
- DOI: 10.3389/fmicb.2022.909109
Reduction Kinetic of Water Soluble Metal Salts by Geobacter sulfurreducens: Fe2+/Hemes Stabilize and Regulate Electron Flux Rates
Abstract
Geobacter sulfurreducens is a widely applied microorganism for the reduction of toxic metal salts, as an electron source for bioelectrochemical devices, and as a reagent for the synthesis of nanoparticles. In order to understand the influence of metal salts, and of electron transporting, multiheme c-cytochromes on the electron flux during respiration of G. sulfurreducens, the reduction kinetic of Fe3+, Co3+, V5+, Cr6+, and Mn7+ containing complexes were measured. Starting from the resting phase, each G. sulfurreducens cell produced an electron flux of 3.7 × 105 electrons per second during the respiration process. Reduction rates were within ± 30% the same for the 6 different metal salts, and reaction kinetics were of zero order. Decrease of c-cytochrome concentrations by downregulation and mutation demonstrated that c-cytochromes stabilized respiration rates by variation of their redox states. Increasing Fe2+/heme levels increased electron flux rates, and induced respiration flexibility. The kinetic effects parallel electrochemical results of G. sulfurreducens biofilms on electrodes, and might help to optimize bioelectrochemical devices.
Keywords: Geobacter sulfurreducens; bioelectrochemistry; c-cytochrome; reaction kinetic; remediation.
Copyright © 2022 Karamash, Stumpe, Dengjel, Salgueiro, Giese and Fromm.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




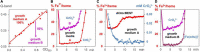

References
-
- Bochdansky A. B., Stouffer A. N., Washington N. N. (2021). Adenosine triphosphate (ATP) as a metric of microbial biomass in aquatic systems: new simplified protocols, laboratory validation, and a reflection on data from the literature. Limnol. Oceanogr. Methods 19 115–131. 10.1002/lom3.10409 - DOI
-
- Bruderer R., Bernhardt O. M., Gandhi T., Miladinović S. M., Cheng L. Y. (2015). Extending the limits of quantitative proteome profiling with data-independent acquisition and application to acetaminophen-treated three-dimensional liver microtissues. Mol. Cell. Proteomics 14 1400–1410. 10.1074/mcp.M114.044305 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

