Structure and Protein-Protein Interactions of Ice Nucleation Proteins Drive Their Activity
- PMID: 35783412
- PMCID: PMC9247515
- DOI: 10.3389/fmicb.2022.872306
Structure and Protein-Protein Interactions of Ice Nucleation Proteins Drive Their Activity
Abstract
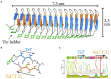
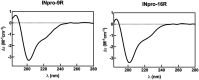
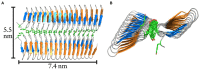
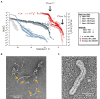
Microbially-produced ice nucleating proteins (INpro) are unique molecular structures with the highest known catalytic efficiency for ice formation. Airborne microorganisms utilize these proteins to enhance their survival by reducing their atmospheric residence times. INpro also have critical environmental effects including impacts on the atmospheric water cycle, through their role in cloud and precipitation formation, as well as frost damage on crops. INpro are ubiquitously present in the atmosphere where they are emitted from diverse terrestrial and marine environments. Even though bacterial genes encoding INpro have been discovered and sequenced decades ago, the details of how the INpro molecular structure and oligomerization foster their unique ice-nucleation activity remain elusive. Using machine-learning based software AlphaFold 2 and trRosetta, we obtained and analysed the first ab initio structural models of full length and truncated versions of bacterial INpro. The modeling revealed a novel beta-helix structure of the INpro central repeat domain responsible for ice nucleation activity. This domain consists of repeated stacks of two beta strands connected by two sharp turns. One beta-strand is decorated with a TxT amino acid sequence motif and the other strand has an SxL[T/I] motif. The core formed between the stacked beta helix-pairs is unusually polar and very distinct from previous INpro models. Using synchrotron radiation circular dichroism, we validated the β-strand content of the central repeat domain in the model. Combining the structural model with functional studies of purified recombinant INpro, electron microscopy and modeling, we further demonstrate that the formation of dimers and higher-order oligomers is key to INpro activity. Using computational docking of the new INpro model based on rigid-body algorithms we could reproduce a previously proposed homodimer structure of the INpro CRD with an interface along a highly conserved tyrosine ladder and show that the dimer model agrees with our functional data. The parallel dimer structure creates a surface where the TxT motif of one monomer aligns with the SxL[T/I] motif of the other monomer widening the surface that interacts with water molecules and therefore enhancing the ice nucleation activity. This work presents a major advance in understanding the molecular foundation for bacterial ice-nucleation activity.
Keywords: atmospheric bacteria; ice-nucleating proteins; protein activity; protein structure; protein–protein interactions.
Copyright © 2022 Hartmann, Ling, Dreyer, Zipori, Finster, Grawe, Jensen, Borck, Reicher, Drace, Niedermeier, Jones, Hoffmann, Wex, Rudich, Boesen and Šantl-Temkiv.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Specific Ion-Protein Interactions Influence Bacterial Ice Nucleation.Chemistry. 2021 May 6;27(26):7402-7407. doi: 10.1002/chem.202004630. Epub 2021 Mar 16. Chemistry. 2021. PMID: 33464680 Free PMC article.
-
Ice-nucleating proteins are activated by low temperatures to control the structure of interfacial water.Nat Commun. 2021 Feb 19;12(1):1183. doi: 10.1038/s41467-021-21349-3. Nat Commun. 2021. PMID: 33608518 Free PMC article.
-
Water-organizing motif continuity is critical for potent ice nucleation protein activity.Nat Commun. 2022 Aug 26;13(1):5019. doi: 10.1038/s41467-022-32469-9. Nat Commun. 2022. PMID: 36028506 Free PMC article.
-
History of Discovery and Environmental Role of Ice Nucleating Bacteria.Phytopathology. 2023 Apr;113(4):605-615. doi: 10.1094/PHYTO-07-22-0256-IA. Epub 2023 Apr 26. Phytopathology. 2023. PMID: 36122194 Review.
-
Overview of biological ice nucleating particles in the atmosphere.Environ Int. 2021 Jan;146:106197. doi: 10.1016/j.envint.2020.106197. Epub 2020 Nov 30. Environ Int. 2021. PMID: 33271442 Review.
Cited by
-
Ice nucleation proteins self-assemble into large fibres to trigger freezing at near 0 °C.Elife. 2023 Dec 18;12:RP91976. doi: 10.7554/eLife.91976. Elife. 2023. PMID: 38109272 Free PMC article.
-
Hierarchical assembly and environmental enhancement of bacterial ice nucleators.Proc Natl Acad Sci U S A. 2024 Oct 22;121(43):e2409283121. doi: 10.1073/pnas.2409283121. Epub 2024 Oct 17. Proc Natl Acad Sci U S A. 2024. PMID: 39418308 Free PMC article.
-
Functional aggregation of cell-free proteins enables fungal ice nucleation.Proc Natl Acad Sci U S A. 2023 Nov 14;120(46):e2303243120. doi: 10.1073/pnas.2303243120. Epub 2023 Nov 9. Proc Natl Acad Sci U S A. 2023. PMID: 37943838 Free PMC article.
-
Expression of Ice Nucleation Protein in Bacillus amyloliquefaciens and Its Application in Food Freezing Process.Foods. 2023 Oct 24;12(21):3896. doi: 10.3390/foods12213896. Foods. 2023. PMID: 37959016 Free PMC article.
-
Protein structure prediction in the era of AI: Challenges and limitations when applying to in silico force spectroscopy.Front Bioinform. 2022 Oct 7;2:983306. doi: 10.3389/fbinf.2022.983306. eCollection 2022. Front Bioinform. 2022. PMID: 36304287 Free PMC article.
References
-
- Amato P., Joly M., Schaupp C., Attard E., Möhler O., Morris C. E., et al. . (2015). Survival and ice nucleation activity of bacteria as aerosols in a cloud simulation chamber. Atmos. Chem. Phys. 15, 6455–6465. doi: 10.5194/acp-15-6455-2015 - DOI
LinkOut - more resources
Full Text Sources

