Antibiofilm and Antimicrobial Activities of Chloroindoles Against Uropathogenic Escherichia coli
- PMID: 35783430
- PMCID: PMC9244173
- DOI: 10.3389/fmicb.2022.872943
Antibiofilm and Antimicrobial Activities of Chloroindoles Against Uropathogenic Escherichia coli
Abstract
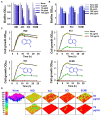
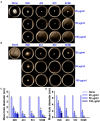
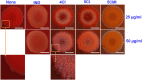
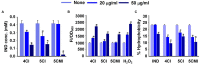
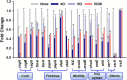
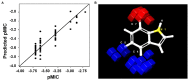
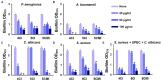
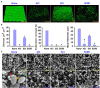
Uropathogenic Escherichia coli (UPEC) is a nosocomial pathogen associated with urinary tract infections and expresses several virulence factors that cause recurring infections and cystitis of the bladder, which can lead to pyelonephritis. UPEC uses different types of extracellular appendages like fimbriae and pili that aid colonization and adherence to bladder epithelium and can form persistent biofilm-like bacterial communities that aid its survival after the deployment of host immune responses. We investigated the antibiofilm, antimicrobial, and antivirulence properties of three indole derivatives namely, 4-chloroindole, 5-chloroindole, and 5-chloro 2-methyl indole. All the three chloroindoles had MICs of 75 μg/ml and inhibited biofilm formation by an average of 67% at 20 μg/ml. In addition, they inhibited swarming and swimming motilities, which are essential for dissemination from bacterial communities and colonization, reduced cell surface hydrophobicity, and inhibited indole production and curli formation. Gene expression analysis showed all three chloroindoles significantly downregulated the expressions of virulence genes associated with adhesion, stress regulation, and toxin production. A 3D-QSAR analysis revealed substitutions at the fourth and fifth positions of the indole moiety favored antimicrobial activity. Furthermore, these chloroindoles potently inhibited biofilm formation in other nosocomial pathogens and polymicrobial consortia.
Keywords: E. coli; UPEC; biofilm; chloroindoles; indoles; virulence.
Copyright © 2022 Boya, Lee and Lee.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Broad-Spectrum Antimicrobial and Antibiofilm Activities of Halogenated Anilines Against Uropathogenic Escherichia coli and ESKAPE Pathogens.Microb Biotechnol. 2025 May;18(5):e70165. doi: 10.1111/1751-7915.70165. Microb Biotechnol. 2025. PMID: 40417767 Free PMC article.
-
Antimicrobial and antibiofilm activities of chromone derivatives against uropathogenic Escherichia coli.Microbiol Res. 2024 Jan;278:127537. doi: 10.1016/j.micres.2023.127537. Epub 2023 Oct 27. Microbiol Res. 2024. PMID: 37922697
-
Antibacterial and Antibiofilm Activities of Chloroindoles Against Vibrio parahaemolyticus.Front Microbiol. 2021 Aug 2;12:714371. doi: 10.3389/fmicb.2021.714371. eCollection 2021. Front Microbiol. 2021. PMID: 34408739 Free PMC article.
-
Virulence factors of uropathogenic E. coli and their interaction with the host.Adv Microb Physiol. 2014;65:337-72. doi: 10.1016/bs.ampbs.2014.08.006. Epub 2014 Nov 4. Adv Microb Physiol. 2014. PMID: 25476769 Review.
-
A systematic review and meta-analysis of antibiotic resistance patterns, and the correlation between biofilm formation with virulence factors in uropathogenic E. coli isolated from urinary tract infections.Microb Pathog. 2020 Jul;144:104196. doi: 10.1016/j.micpath.2020.104196. Epub 2020 Apr 10. Microb Pathog. 2020. PMID: 32283258
Cited by
-
5-Methylindole kills various bacterial pathogens and potentiates aminoglycoside against methicillin-resistant Staphylococcus aureus.PeerJ. 2022 Sep 14;10:e14010. doi: 10.7717/peerj.14010. eCollection 2022. PeerJ. 2022. PMID: 36124131 Free PMC article.
-
Broad-Spectrum Antimicrobial and Antibiofilm Activities of Halogenated Anilines Against Uropathogenic Escherichia coli and ESKAPE Pathogens.Microb Biotechnol. 2025 May;18(5):e70165. doi: 10.1111/1751-7915.70165. Microb Biotechnol. 2025. PMID: 40417767 Free PMC article.
-
Synthetic and Semisynthetic Compounds as Antibacterials Targeting Virulence Traits in Resistant Strains: A Narrative Updated Review.Antibiotics (Basel). 2023 May 25;12(6):963. doi: 10.3390/antibiotics12060963. Antibiotics (Basel). 2023. PMID: 37370282 Free PMC article. Review.
-
Molecularly Imprinted Electrochemical Sensors for Halogenated anti-Infective Agent Detection: A Review of Current Developments and Prospects.ACS Omega. 2025 Aug 7;10(32):35327-35351. doi: 10.1021/acsomega.5c03269. eCollection 2025 Aug 19. ACS Omega. 2025. PMID: 40852300 Free PMC article. Review.
-
Impact of halogenation on scaffold toxicity assessed using HD-GEM machine learning model.Brief Bioinform. 2025 Jul 2;26(4):bbaf347. doi: 10.1093/bib/bbaf347. Brief Bioinform. 2025. PMID: 40675561 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

